|
I. The Benzannulation Reaction of
Unsaturated Complexes with Alkynes. |
||
|
Carbene Complexes I. Benzannulation Reaction II. Cyclohexadienone Annulation III. Tautomer Arrested Annulation VIII. Macrocycles
I. Ligand Design and Synthesis II. Asymmetric Diels-Alder Reaction III. Imino Aldol Reaction |
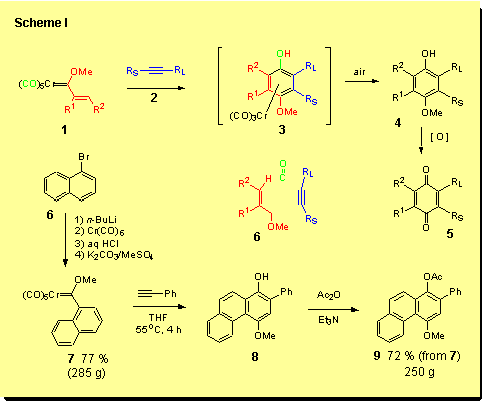
The reaction of Fischer carbene complexes that has grown to greatest prominence in applications in organic synthesis is the benzannulation reaction. This is a truly amazing reaction that was discovered by Karl Heinz Dötz and involves the reaction of unsaturated complexes with alkynes to give 4-alkoxyphenols of the type 4 as the primary product of the reaction.The initial product of the reaction is 3, the corresponding arene chromium tricarbonyl complex of the phenol 4, but these typically are unstable to air such that workup in air and purification of the product on silica gel leads to the complete loss of the metal.The phenol 4 is the result of the assembly of the alkyne, the carbene ligand and a carbon monoxide ligand in the coordination sphere of the metal. That this highly orchestrated process occurs under neutral conditions and near ambient temperatures (45 oC) to typically produce high yields of the phenol products has never ceased to be a source of amazement and inspiration.
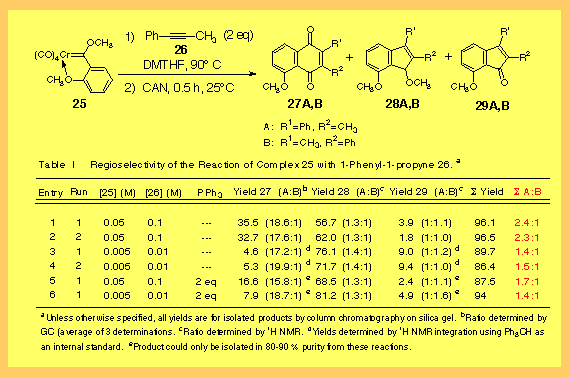
With unsymmetrical acetylenes mixtures of products are usually obtained where the ratio normally correlates with the steric differential between the two substituents [1]. The largest group (RL) is incorporated adjacent to the carbon monoxide derived carbon. Terminal alkynes are usually highly regioselective with selectivities typically of 100 : 1 or more. This reaction can produce a wide variety of phenol products. The reaction of vinyl complexes generate phenols, that of aryl complexes gives naphthols and heteroaryl complexes can give a variety of benzannulated heterocycles. This reaction is of enormous synthetic importance not only for its utility in the synthesis of highly substituted phenols but also as a result of the further conversion of these phenols to quinones. Quinones are ubiquitous subunits that occur in a vast variety of biological active natural products and pharmaceutical agents. Despite the extensive study that has been devoted to this reaction over the last twenty-eight years, much remains to be learned about this reaction. We have recently completed a comprehensive review of this reaction and the Table containing all of the known examples of this reaction is over 500 pages long [2]. |
|
|
Synthesis of Natural Products and Pharmaceuticals
|
|
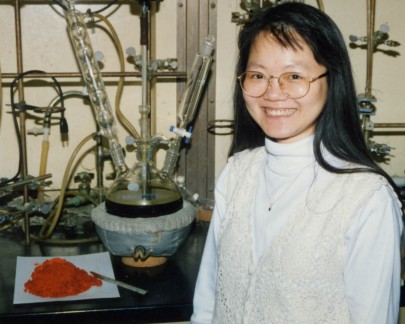
Typically, Fischer carbene complexes are solids which means their preparation and reactions can be performed on open ended scale since purification can be accomplished by crystallization rather than by chromatography. One example that we have scaled up is the preparation and reaction of the naphthalene complex 7.This complex was prepared from 1-bromonaphthalene on a 285 gram scale in 77 % yield (3 crops). The reaction of naphthyl complex 7 with phenylacetylene was carried out in THF to give the phenanthrol 8 which was not isolated, but directly acetylated to give the acetate 9 on a 250 gram scale in 72 % overall yield for the two steps. In the photograph, Eliza Yeung is performing the benzannulation of complex 7 with phenylacetylene. The red pile is 250 grams of the naphthalene complex 7 and in the flask is another 250 grams of the complex reacting with phenylacetylene. This particular reaction is used in the synthesis of the VAPOL ligand and its chemistry will be discussed in the section on asymmetric catalysis. |
|
[1] For recent citations to the literature, see: Wang, H.; Wulff, W. D.; Rheingold, A. L.; J. Am. Chem. Soc., 2000, 122, 9862. [2] Waters, M. L.; Wulff, W. D., Organic Reactions, in press.
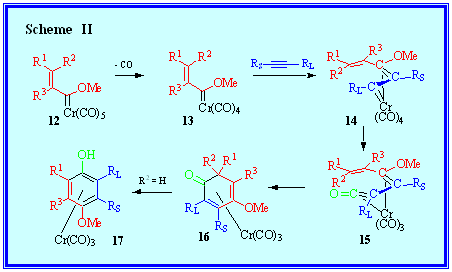
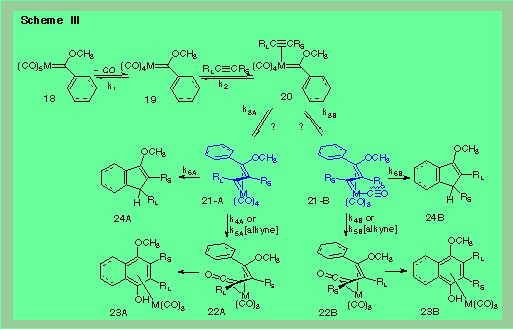
I-a:The mechanism of the benzannulation reaction. |
||