|
IV.
A General Asymmetric
Catalytic Aziridination Reaction (AZ).
|
There
have been a number of successful chiral catalysts that have been
developed for the asymmetric synthesis of epoxides.
The same is not true for epoxide’s aza cousins,
aziridines. When we
became interested in this subject in 1996 there were no catalytic
asymmetric aziridination reactions known that worked for a variety
of substrates. We
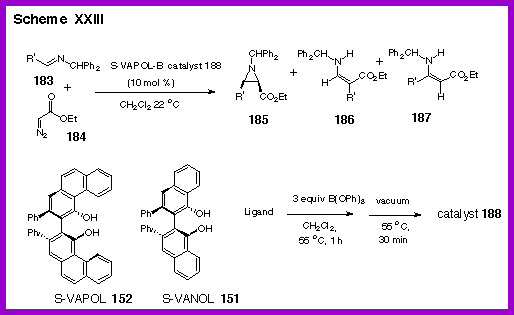
became interested in the synthesis of aziridines from the
reactions of imines with diazo compounds mediated by a Lewis acid.
The first report of general method
for the generation of an aziridine from the
reaction of an imine and ethyl diazoacetate with simple
Lewis
acids appeared in 1996 by Brookhart and
Templeton. |
|
|
Inspired
by the work of Brookhart and Templeton, we decided to attempt to
render this reaction asymmetric by the use of a chiral Lewis acid
prepared from the VANOL or VAPOL ligand [1]
The aluminum catalyst that we had found to be effective in
the asymmetric Diels-Alder reaction described above was only
moderately successful. After
considerable experimentation with other Lewis acids in combination
with VAPOL we eventually found that an extremely effective and
general catalyst could be prepared from triphenylborate and VAPOL.
The catalyst is prepared by heating VAPOL and
triphenylborate at 55 oC for one hour and then removing
all volatiles at this same temperature under vacuum.
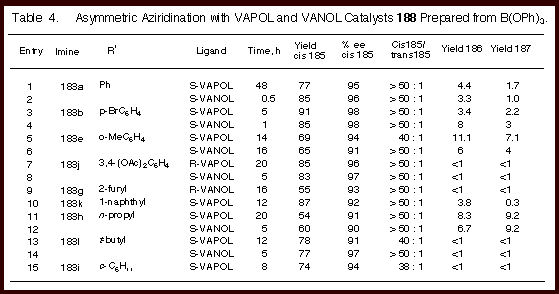
As indicated by the data in Table 4, the reaction is highly
diastereoselective for the formation of cis-aziridines with up to
≥ 50 : 1 cis to trans selectivity and in 55 - 91 % isolated
yields for nine different imine substrates. The enantioselectivity is remarkably high (90 - 98 % ee) over
a range of imines which include those prepared from electron-poor
and electron-rich aryl aldehydes and from branched and unbranched
aliphatic aldehdyes. The
reaction is rapid giving 20 turnovers an hour at room temperature
for the imine from benzaldehyde which provides the aziridine 185a.
The reaction can be catalyzed with as little as 0.5 mole
percent catalyst to give 185a
in 80 % yield and 98
% ee after 24 hours.
It
was remarkable to find that catalysts derived from both VANOL and
VAPOL are effective in this reaction.
The variation between the two catalysts is quite small. In all other reactions that we have examined to this point,
the VANOL and VAPOL ligands have always led to disparate levels of
induction. In the
present case, the asymmetric inductions track nearly identically
with each substrate and only small differences are seen. For
some reactions slightly higher inductions were posted by the VANOL
catalyst and for others the higher induction was observed with the
VAPOL catalyst. What is remarkable is that for both ligands and for all
substrates, the asymmetric induction for all combinations falls in
the range of 90 – 98 % ee. The diastereoselectivites are very
high for both catalysts but the VANOL catalyst gave a selectivity
of greater than 50 : 1 for every substrate.
|
|
Based
on the information that we have at this time, our current working
model assumes the formation of a one to one adduct of the VAPOL
ligand and triphenyl borate with two of the phenoxy groups
replaced by the phenol functions of the VAPOL ligand. We have
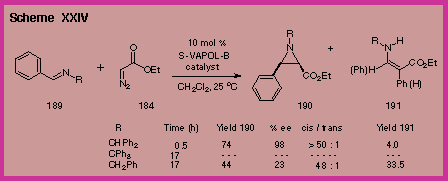
found that the benzhydryl substituent on the imine is crucial.
With the catalyst generated from VAPOL we found that the
benzhydryl imine 189
was far superior to the benzyl imine in terms of yield, rate and
asymmetric induction (Scheme XXIV).
The trityl imine was unreactive, presumably due to steric
hindrance in the approach to the chiral VAPOL boron complex. |
|
|
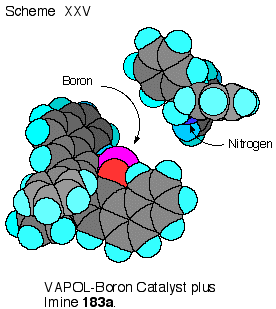
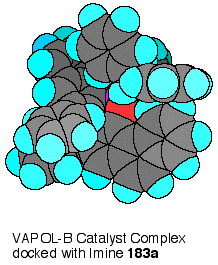
The
benzhydryl imine 189 (R
= CHPh2) has two low energy conformations which are
enantiomers that differ by the direction of the twist of the
phenyl groups in the diphenylmethyl substituent.
One of these enantiomers can dock with the VAPOL ligand to
give two face- edge arene interactions.
This is shown in Scheme XXV where one phenyl ring of the
imine has its edge in contact with the face of one of the
phenanthrene rings of VAPOL, whereas, the other phenyl ring of the
imine has its face up against the edge of the other phenanthrene
ring of the VAPOL ligand. For
benzene, the face-edge interaction is more energetically favorable
than face-face interaction and it has been estimated that in
solution this interaction is worth about 0.7 Kcal mol-1.
|
You can see 3D
structure of 183.MOL (then click with right mouse button
on 3D wireframe image and choose "display
option" in menu to get the different space models) with
the help of MDL Chime plug-in (free download here)
|
|
Two of these interactions could well account for the large
difference between the benzyl imine 189
(CH2Ph) and the benzhydryl imine 189
(CHPh2). CPK
models reveal that similar interactions are observed for the VANOL
ligand but not for BINOL, which gives very low inductions for this
aziridination reaction. We
are currently testing this model for the asymmetric induction in
the aziridination reaction. We
are also examining the scope of this reaction with other diazo
compounds and imines as well pursuing the applications of this
catalytic asymmetric aziridination (AZ) in organic synthesis.
[1]
Antilla, J.; Wulff, W. D. Angew.
Chem. Int. Ed. Engl., 2000,
39, 4518.
|
|
|
Enantioselective Catalysis
I. Design and
Synthesis of VANOL and VAPOL
II. Organometallic Catalyst
III. Organocatalysts
1. Asymmetric
Aziridination
2. Heteroatom Diels-Alder Reaction
3. Aza-Cope Reaction
4. Aza-Henry Reaction
5. Nitroalkane/Nitroalkene Addition
Total Synthesis of Natural Products
|




