|
III.
Active Site Design in a
Chemzyme. A Catalyst for
the Imino Aldol Reaction
|
The
asymmetric aldol reaction of an enolate or enolate equivalent with
an imine is a reaction of established synthetic importance for the
synthesis of chiral amines in general and b-amino esters in
particular. The first useful catalyst for this reaction was
reported by Kobayashi and coworkers.
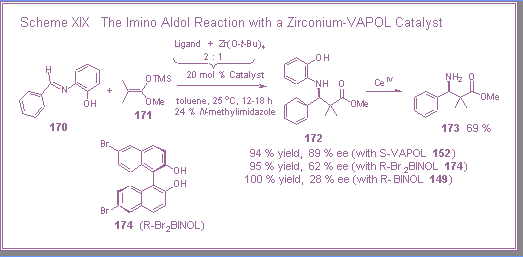
Their method involves the reaction of an imine derived from
o-aminophenol and a ketene acetal with a catalyst derived from
zirconium tetra-t-butoxide
and two equivalents of R-6,6’-dibromoBINOL 174
(Scheme XIX). |
|
|
Our
interest in the synthesis of chiral amines led us to investigate
the use of a VAPOL derived catalyst for this reaction.[1]
Kobayashi reported that the catalyst derived from the
dibromoBINOL ligand 174
gives good asymmetric induction (87 % ee) for the reaction of
imine 170 and ketene
acetal 171 at –45 oC.
Remarkably, we found that a catalyst prepared from the
VAPOL ligand and zirconium tetra-t-butoxide could give high asymmetric induction even at room
temperature for the same reaction.
The induction from the catalyst prepared from the
dibromoBINOL ligand 174
fell to 62 % ee when the temperature was raised to room
temperature. |
|
|
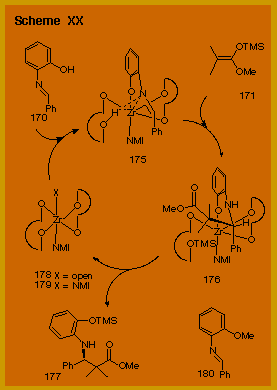
A mechanism to account for the catalysis of the reaction of imine 170
with ketene acetal 171
is shown in Scheme XX.
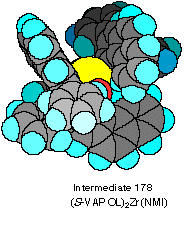
It is clear from the examination of space filling CPK
models that it is possible to bind two VAPOL ligands to one
zirconium atom but only with a facial arrangement of the four
oxygen atoms as is illustrated by the structure 178 in Scheme XX. This
is supported by 1H NMR experiments on a catalyst
generated from zirconium tetra-iso-propoxide
and VAPOL in the presence of two equivalents of N-methyl
imidazole. A clean
spectrum is only observed with two equivalents of VAPOL relative
to zirconium and the spectrum is consistent with a single C2-symmetrical
species, which is tentatively identified as structure 179 bearing mutually trans
NMI ligands bound to the zirconium.
An approach of imine 170
to the open apical position in intermediate 178
is proposed to lead to intermediate 175
in which a phenol exchange has occurred.
In support of this is the observation that the catalysis of
the reaction of the O-methylated imine 180
with acetal 171 under
the conditions in entry 3 in Table 2 gave 173
in 5 % yield and 0 % ee.
The requirement for NMI then can be explained as that of a
mono-dentate ligand that binds to the other apical site and
maintains an octahedral geometry. Reaction of species 175 with the ketene acetal would give intermediate 176
and then release of the product would regenerate the unsaturated
species 178 and
complete the cycle.
|
|
|
A space filling CPK
model of intermediate 178
is shown in Scheme XXI and illustrates the binding cleft
that is available for the docking with the imine 170.
There are a number of possible orientations of the imine 170
in the cleft of 178 and
these are largely associated with changes in the Zr-O-C bond angle
that has the effect of rocking the imine back and forth in the
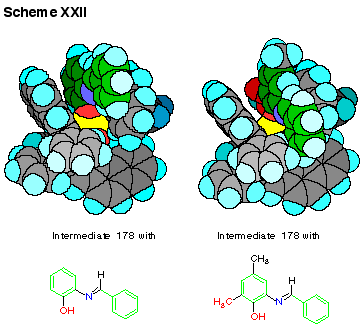
cleft. Rocking the
imine down into the cleft should provide a conformation that
provides a greater facial selectivity of attack on the imine since
it should provide greater shielding of the re-face by the
phenanthrene unit on the right side of the molecule as viewed in Scheme
XXII. CPK models
reveal that a methyl group on the imine ortho to the phenol
function should be sufficient to push the imine down into the
cleft. This methyl
group is presented in red in the imine complex with intermediate 178
and as illustrated in Scheme XXII this methyl group makes
close contacts with the floor of the cleft even when the imine is
rotated down into the cleft and this results in greatly restricted
movement about the zirconium-oxygen-carbon bond to the imine unit.
This model thus predicts that imines with a methyl
substituent ortho to the phenol function in imine 170
should lead to increased asymmetric induction in the imino aldol
reaction and methyl groups at positions meta and para to the
phenol should not have any effect since they do not make close
contacts with any part of the catalyst. |
|
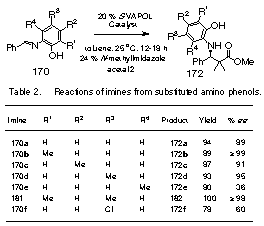
On
the basis of the above predictions, a series of seven
substituted imines were prepared and data from their
reactions with ketene acetal 171
are summarized in Table 2. Indeed,
of the four possible mono-methyl substituted imines, the
highest induction was observed with a methyl group ortho
to the phenol function (R1 = Me).
The asymmetric induction increases from 89 % ee
with imine 170a to > 99 % ee
with imine 170b
(entries 1 and 2). The
induction significantly drops with imine 170e
with a methyl group ortho to the imine and this may due to
twisting of the imine to expose the re-face. The introduction of a methyl group at R2 has very
little effect and the slight increase in induction for the
methyl group at R3 may indicate an electronic
effect that results in a shortening of the
zirconium-oxygen.
This is corroborated with the decrease in induction
observed for the imine 170f with a chloro substituent at R3.
The catalyst generated from 6,6’-dibromoBINOL 174
has a completely different response to substituents on the
imine; the induction drops with introduction of a methyl
group ortho to the phenol. The catalyst prepared
from this ligand gives a 62 % ee
with imine 170a
and a 47 % ee
with the dimethyl substituted imine 181. |
You can see 3D structures of
178 (178a.MOL
and 178b.MOL) and display
them (just right mouse button click on 3D wireframe image)
as spacefill or stick models using MDL Chime plug-in (for free download click here). |
|
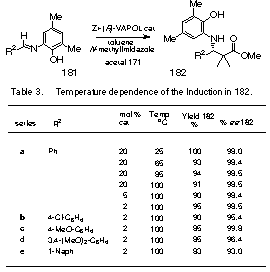
The rate of the reaction of imines
with ketene acetals with the VAPOL catalyst is slower with imines
generated from substituted aminophenols, however, as indicated in Table 3
this effect can be offset by performing the reaction at higher temperature
where greater turnover numbers are observed.
It was quite striking to observe that the induction with imine 181a
shows absolutely no temperature dependence over the range of 25 to 100 oC.
Furthermore, the
reaction at 100 oC can be performed with an order of magnitude
change in catalyst loading with no loss in induction.
The turnover numbers have not yet been measured, as the minimum
time for these reactions was not investigated.
The electronic nature of the imine has a small effect on the
induction at this temperature with a slight increase noted for a p-methoxy
substituent and slight decrease for a p-chloro
substituent. Further studies
concerning the mechanism of this catalytic process are ongoing.
[1]
Xue,
S.; Yu, S.; Deng, Y.; Wulff, W. D., Angew.
Chem. Int. Ed. Engl., 2001,
40, 2271.
|
Asymmetric
Catalysis
I. Ligand Design and
Synthesis
II. Asymmetric Diels-Alder
Reaction
III. Imino Aldol Reaction
IV. Asymmetric
Aziridination
Fisher
Carbene Complexes
Introduction
I.
Benzannulation
Reaction
II.
Cyclohexadienone
Annulation
III. Tautomer
Arrested Annulation
IV. Aldol Reaction
V. Diels-Alder Reaction
VI. Cyclobutanone
Formation
VII. Biaryl Synthesis
VIII.
Macrocycles
Synthesis of Natural Products
and Pharmaceuticals
|