|
I.
Design and Synthesis of VANOL and VAPOL
Our
entry into the field of asymmetric catalysis began with a very simple
idea. BINOL is one of the most widely used chiral ligands and this is
because of the wide range of reactions for which it is effective.
Nonetheless, it certainly does not work well for all reactions and it
does not work well for all substrates for those reactions that it is the
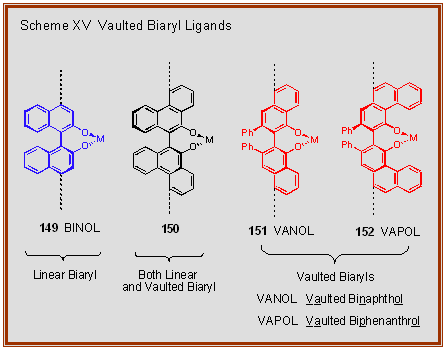
ligand of choice. Scheme XV depicts a BINOL ligand bound to
either a transition metal or a main group metal. The metal is bound to
the phenolic groups in the 2 and 2’ positions of the BINOL and the
reaction that takes at the metal center is rendered asymmetric by the
chirality of the ligand.
|
|
It can be seen
that the bulk of the space that is asymmetrically discriminated
by the BINOL ligand is on the side of the chiral axis that is
opposite that where the reaction is taking place at the metal
center. Thus, the simple idea was to move the annulated benzene
rings from the back of BINOL to the front to give a molecule of
the type 151, which we call VANOL. It was necessary to
add phenyl groups at the 3 and 3’-positions to allow for
resolution. Space filling models reveal that there is a deeper
chiral pocket surrounding the metal center in VANOL than in
BINOL. Addition of an extra set benzene rings onto the front of
VANOL gives 152 (VAPOL). a molecule with an even deeper
chiral pocket around the metal center. The names VANOL and VAPOL
are derived from the fact that both compounds are members of the
family of vaulted biaryls that have annulated benzene rings
curving around the nascent active site of the ligand-metal
complex.
|
|
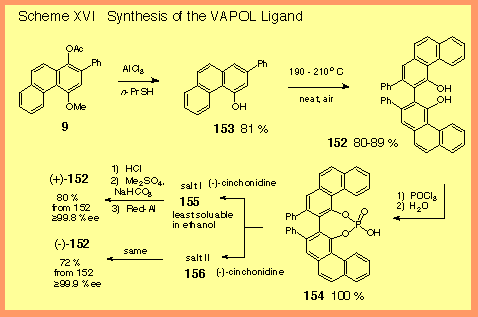
The
syntheses of the VANOL and VAPOL ligands are similar and are
illustrated by the synthesis of the VAPOL ligand shown in Scheme
XVI [1]. The synthesis begins
with the protected phenanthrol 9 which was prepared from
the benzannulation of the naphthalene carbene complex 7
with phenylacetylene as indicated in Scheme
I. Borrowing from an
esoteric reaction, we were able to reduce the acetate and cleave
the methyl ether in a single step with aluminum chloride and
propanethiol to give the phenanthrol 153 in 81 % yield on
scales up to 300 grams. The oxidative coupling of the
phenanthrol 153 failed with traditional methods involving
metal oxidants, but was successful simply by heating in air.
This was done neat by melting 153 at 190 –210 oC
in the presence of air in a test tube. On bigger scale, the
optimal temperature was 185 oC or 365 oF. |
|
|

|
As
indicated in the photograph, Dr. Eugene Grant found that the
best method for large scale synthesis was to load a lasagna pan
with a thin layer of 153 (40 – 80 g) and bake it in an
oven at 365 oC for 24 hours. The resolution is
carried out in a classical manner involving selective
crystallization of diastereomeric salts. The salts were prepared
by initial conversion of racemic VAPOL 152 to the cyclic
phosphonic acid 154. Crystalline salts of this acid were
obtained by the addition of (-)-cinchonidine. The resolution was
made even easier by the fact that both diastereomeric salts were
crystalline. After the first salt was collected, the solvent was
removed and then crystallization gave the second salt.
Liberation of VAPOL from each salt gave access to both
enantiomers of VAPOL in optically pure form. We have recently
found that the resolution can be avoided by employing a
deracemization procedure involving copper (II) salts and (-)-spartiene
[2]. |
[1]
Bao,
J.; Wulff, W. D.; Dominy, J. B.; Fumo, M. J.; Grant, E. B.; Rob, A. C.;
Whitcomb, M. C.; Yeung, S.-M.; Ostrander, R. L.; Rheingold, A. L., J.
Am. Chem. Soc., 1996, 118, 3392.
[2]
Zhang,
Y.: Yeung, S.-M.; Wu, H.; Heller, D. P.; Wu, C.; Wulff, W. D., Org.
Lett., 2003, 5, 1813.
|
Enantioselective Catalysis
I. Design and
Synthesis of VANOL and VAPOL
II. Organometallic Catalysts
III. Organocatalysts
|


