|
Carbene
Complexes
Introduction
I. Benzannulation
Reaction
II.
Cyclohexadienone
Annulation
III. Tautomer
Arrested Annulation
IV. Aldol
Reaction
V. Diels-Alder
Reaction
VI. Cyclobutanone
Formation
VII. Biaryl Synthesis
VIII.
Macrocycles
Asymmetric
Catalysis
I. Ligand Design
and
Synthesis
II. Asymmetric
Diels-Alder
Reaction
III. Imino Aldol
Reaction
IV. Asymmetric
Aziridination
Synthesis of Natural Products
and Pharmaceuticals
|
|
We
have recently published an initial study on a new method for the
construction of biaryl chromium tricarbonyl complexes [1].iThe
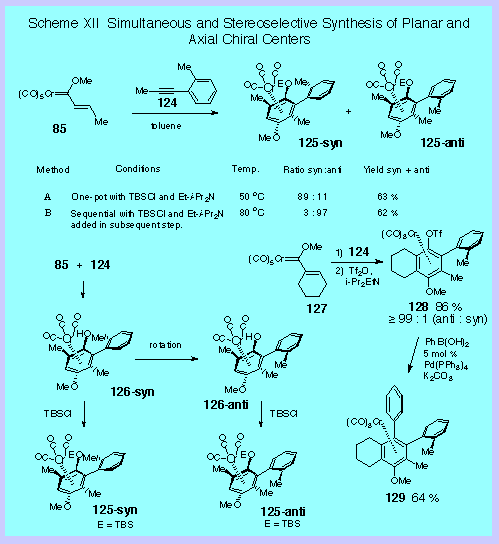
basic concept is illustrated in Scheme XII by the reaction of
complex 85 and the
ortho-substituted aryl acetylene 124. The
question is whether the newly installed planar chiral center
that results from the coordination of the chromium tricarbonyl |
|
unit
to the benzene ring formed in the benzannulation reaction can
control the configuration about the axial chiral center that is
also formed at the same time.
If the reaction is carried in the presence of TBSCl and
Hunig’s base the syn-isomer of 125
can be obtained with an 89 : 11 selectivity.
If the benzannulation reaction is performed first and
then the silylation is carried out in a subsequent step, the
anti-isomer can be obtained with a 97 : 3 selectivity.
The explanation is that the syn phenol complex 126
is kinetically favored and is trapped quickly in the presence of
TBSCl to give the syn-arene complex 125.
In the absence of TBSCl, the syn isomer undergoes
isomerization to the more stable anti phenol complex 126
which then can be trapped after the benzannulation is complete.
The scope of this reaction was studied in considerable
detail. Six different alkynes where studied along with four
different carbene complexes.
While most reactions did not give high kinetic
selectivity for the syn isomer, all of the reactions examined
gave high selectivity for the anti isomer with a minimum
selectivity of 94 : 6. It
was also found the phenol complex formed in these reactions
could also be trapped to give aryl triflate chromium tricarbonyl
complexes. This has
considerable |
|
|
synthetic
appeal since, as illustrated for the Suzuki coupling of complex 128,
it provides a method for the easy preparation of more highly
functionalized biaryl systems.
Applications of this process in the synthesis of new
ligands for catalytic asymmetric synthesis are underway.
|
|
The
asymmetric benzannulation was discussed above (Scheme
IV) and provides for a method of asymmetric
induction in the transfer of chirality to the new center
of planar chirality that is produced in the arene
chromium tricarbonyl products of this reaction.
In the asymmetric cyclohexadienone annulation (Scheme
VI) the asymmetric transfer terminates in the
formation of a center of chirality at a ring carbon in
the cyclohexadienone product.
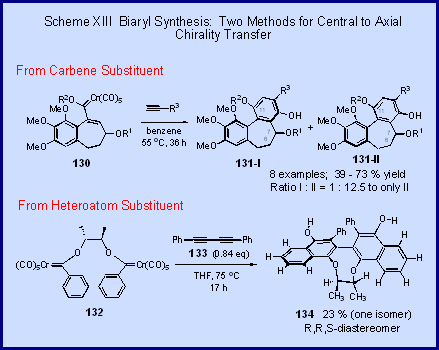
Another potentially very useful mode of chiral
transfer in the benzannulation reaction is that which
terminates in the formation of a new chiral biaryl axis.
We have presently investigated two variations of
this as illustrated in Scheme XIII. In the benzannulation of carbene complexes of the type 130,
which has a chiral center in the carbene carbon
substituent, a high level of asymmetric induction is
observed in the newly formed chiral axis in 131
[2].[Excellent
stereoselection is seen in
the preferred formation of the diastereomer 131-II.
This reaction is being exploited in the total
synthesis of (-)-colchicine.
|
|
The other example involves the transfer of a
center of chirality contained in the heteroatom-stabilizing
group of the carbene complex to a newly formed axial
center. The
reaction of 132
with the diyne 133 occurs with a double-benzannulation to give the biaryl 134
with a complete asymmetric transfer to the chiral axis [3].[Only the S-configuration about
the biaryl bond is formed.
This is a truly unique method for the synthesis
of biaryls since the construction of the bond connecting
the two aryl rings precedes the construction of either
of the aryl rings. This
reaction has tremendous potential for the synthesis of
chiral biaryls for use in asymmetric catalysis.
|
[1]
Fogel, L.; Hsung, R. P.; Wulff, W. D.; Sommer,
R. D.; Rheingold, A. L., J.
Am. Chem. Soc., 2001, 123, 5580.
[2]
Vorogushin, A.
V.; Wulff, W. D.; Hansen, H.-J., J.
Am. Chem. Soc., 2002,
124, 6512.
[i[3]
Bao, J.; Wulff, W. D.;
Fumo, M. J.; Grant, E. B.; Heller, D. P.; Whitcomb, M. C.;
Yeung, S.-M., J. Am.
Chem. Soc., 1996,
118, 2166.
|
|

