|
VIII. Construction of Cyclophanes and other Macrocycles |
|
|
Carbene Complexes II. Cyclohexadienone Annulation III. Tautomer Arrested Annulation VIII. Macrocycles
I. Ligand Design and Synthesis II. Asymmetric Diels-Alder Reaction III. Imino Aldol Reaction
|
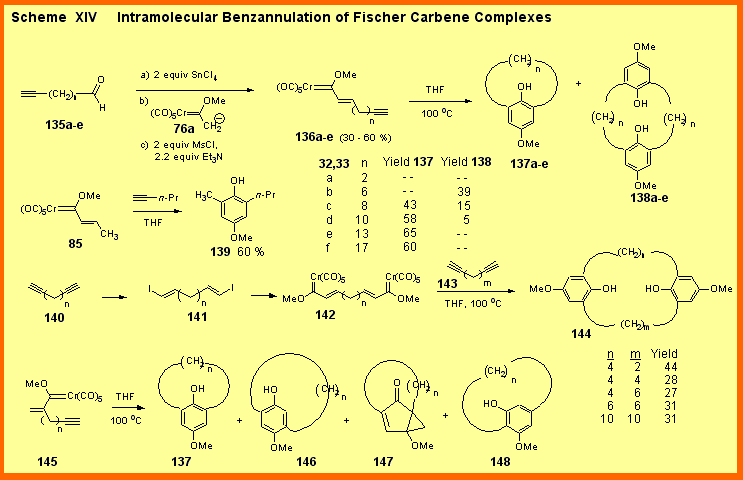
Macrocyclization reactions via the intramolecular benzannulation have curiously not been studied to any significant extent until recently. We have recently examined the intramolecular benzannulation of vinyl carbene complexes of the type 136 [1].[iThe ability to study these reactions depended on the development of the aldol reaction of carbene complexes with alkynals of the type 135 and this work is described above. The optimal temperature for the intramolecular cyclization of 136 is 100 oC and provides a mixture of monomers, dimers and trimers. For the series of complexes 136a - 136f, the trimer (not shown) was never obtained in more that 10-15 %. For the carbene complex 136b with six methylene spacers (n = 6) the dimer 138b is the major product. For all complexes with n ≥ 8 the monomer 137 is the major product. The yield of the monomer is 58 % with n= 10 and no significant change in yields is observed as the chain is increased to n = 17. This can be compared with the intermolecular reaction of complex 85 with 1-pentyne, which gives the phenol 139 in 60 % yield. Thus above n = 10, the intramolecular nature of the reaction seems to have very little effect. In an effort to provide dimers of the type 138 for larger ring sizes we have developed a crossed macrocyclization that involves the reaction of the bis-carbene complex 142 with the diyne 143. While the yields are only modest, dimers of the type 144 can be obtained in yields that do not vary as the n and m values change from 4 to 10. More importantly, this crossed macrocyclization allows for the preparation of unsymmetrical molecules (n ≠ m).
Recently, we have published studies of the macrocyclization of complexes of the type 145 in which the tether is attached to the a-vinyl carbon of the carbene complex [2]. This reaction proved to be full of surprises. First we were startled to find that the a-tethered complex 145a (n = 10) gives the same product as the b-tethered complex 136d (n = 10): m-cyclophane 137d. The expected p-cyclophane 146a was only produced in 4 % yield. The solution to this puzzle was that a totally unprecedented product from the reaction of Fischer carbene complex was also obtained, the cyclopentenone 147. Cyclophane 137 is a secondary product of the reaction resulted from the acid catalyzed opening of 147. Recently, we have found that in benzene the thermolysis of 145 also gives the resorcinol cyclophane 148 [3].[iA meta-alkoxyphenol has never been seen before from the reaction of a carbene complex and an alkyne and thus its structure was confirmed by X-ray. The formation of this product was completely unexpected and puzzling since it requires the breaking of the carbon-carbon bond of the vinyl group in complex 145. The study of the mechanism for the formation of this product is underway. The study of these macrocyclizations has proved to be very fruitful in uncovering many new aspects of the reaction of Fischer carbene complexes with alkynes. The applications of these reactions to the synthesis of calixarenes and related molecules are currently ongoing. |