SYNCHROTRON |
 |
Description of Services
The Center for Crystallographic Research has been in collaboration with the Macromolecular LS-CAT team for data collection for several years. The following provides information regarding the various services offered.
User Policies & Safety
Any member of the MSU can make use of the facility. Most users either collaborate with Dr. Richard J Staples or Professor James Geiger. Safety is controlled by APS, EHS, Department of Chemistry and the respective mangers of the Center.
Synchrotron Small Molecule at Life Sciences Collaborative Access Team (LS-CAT)

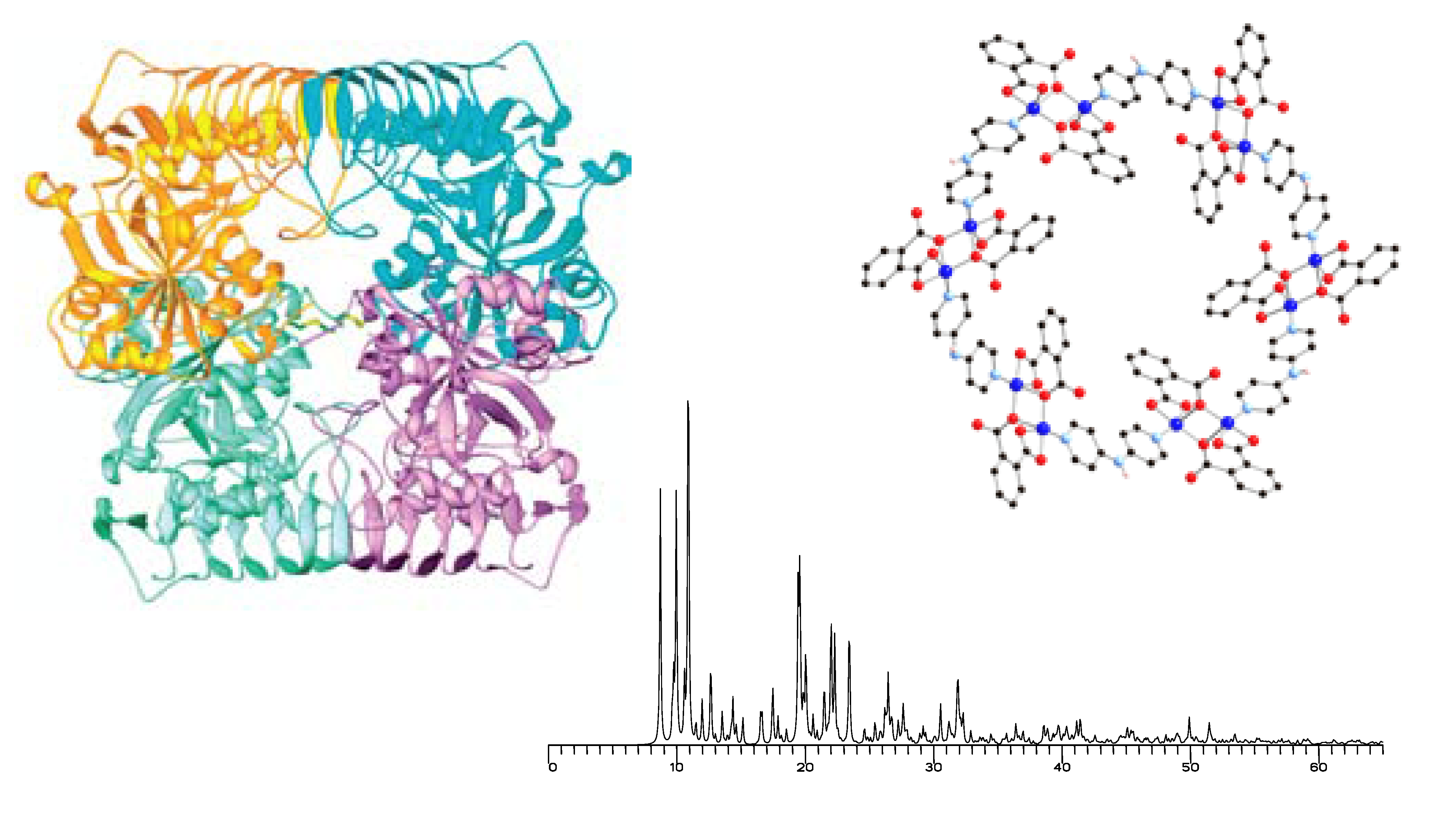
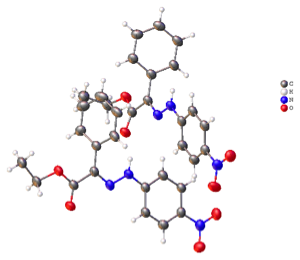
Over the past year the CCR has been making use of time from the LS-CAT program to run small molecule crystal structures on the beam line, 21-D. Although there are some limitations to using this line, it has proven to be very valuable once some standard practices and data collection, software analysis was worked out to produce good data.
Borhan, McCusker, Odom, and Wulff are a few of the faculty at MSU. This is a tremendous step forward and expansion to other research groups within MSU has begun. This is particularly true in the chemistry where smaller crystals or poor diffraction occurs at higher diffraction angles; occur more often such as, Plant sciences, natural products and pharmaceutical compounds.
This facility participated in a recent grant proposal which will improve the facilities in many ways, but significantly for small molecules.
This access is invaluable to the facility now and in the future.
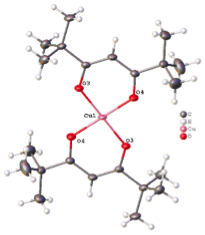
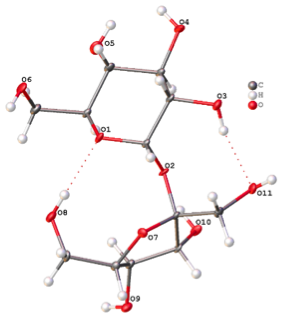
Current dates of Synchrotron data collection:
MSU 21-ID-F Dec 00, 2025 at 10:00am CDT GEIGER
Please note: Crystals must be in hand 4 business days before the assigned time and email sample safety issues 12 days before sending crystals to ensure satisfying all the safety concerns at the APS beam line. ONLY TIME ON D Line works for small molecules.
Macromolecular Crystallography
MSU Chemistry
Protein and large molecule crystallography


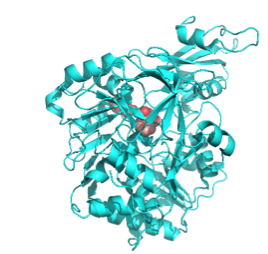
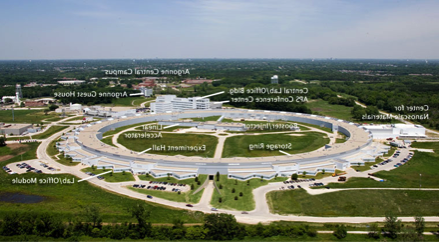
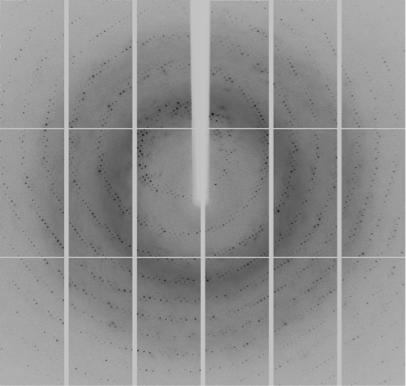
The Life Sciences Collaborative Access Team (LS-CAT) formed in 2002 provides macromolecular crystallography resources for those with a need to determine the structure of proteins. LS-CAT provides access to state-of-the-art x-ray diffraction facilities at Argonne National Laboratory's Advanced Photon Source where extremely intense beams of x-rays are focused using both mirrors and beryllium lenses onto tiny protein crystals. The x-rays diffracted by these crystals are collected with giant CCD detectors that produce the images needed to calculate where the atoms reside in the protein crystal.
More information on LS-CAT go to https://ls-cat.org/index.html.
To use this facility contact Professor James Geiger.











- SCXRD
- Single Crystal X-Ray Diffraction

- PWXRD
- Powder X-Ray Diffraction

- XRF
- X-Ray Fluorescence Spectroscopy

- In-House Protein
- Data Collection