Project Members
Hydridic-Protonic Hydrogen Bonding

Project Overview
In work started in summer of 1994, and primarily
carried forward by summer high school students and undergraduates, we
demonstrated an interaction between the electron pair of a B-H bond and
a traditional H-X (X=N, O, Halogen) hydrogen bonding partner. Single crystal
X-ray and neutron diffraction, solution spectroscopies (IR, NMR), and ab
initio calculations all indicate that such relationships are best understood
as a novel type of hydrogen bonding.
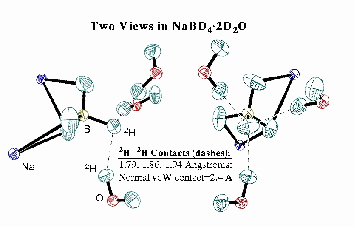
Two views of a segment of the single crystal neutron diffraction structure of NaBD4.2D2O are shown at the top. This structure was obtained by Dr. Rui Huang using the LANSCE facility at Los Alamos in collaboration with Drs. Juergen Eckert, Dimitri Argyriou, and Robert Sheldon. X-ray structures, also taken by MSU's Dr. Huang, had initially revealed not one, but three (!) close hydridic-to-protonic interactions in this hydrate, but the neutron structure confirmed their striking shortness, more than 1/2 Angstrom inside the conventional 2.4 Angstrom estimated van der Waals contact distance. These findings, even on their own, suggest that these interactions are energetically favorable and, like traditional H-bonds, have specific orientational preferences.
While our own studies have focused on the borohydrides,
related examples of close interactions between hydridic and protonic hydrogen
sites in organoiridium complexes have been reported from the Crabtree group
at Yale. [Crabtree, R. H.; Siegbahn, P. E. M.; Eisenstein, O.; Rheingold,
A. L.; Koetzle, T. F. Acc. Chem. Res. 1996, 29, 348-354.]
The new associative effect has implications for regiochemical control of organic reactions and for the rational assembly of crystalline, covalent materials. Consistent with calculations (shown at left) Sterling Gatling has uncovered strong, controllable directing and rate effects in borohydride reduction of hydroxyketones under appropriate conditions.[50] The idea of chemical "basting stitches"-weak linkages that can organize and hold a structure's form while it is more firmly sewn together-is also very appealing. Hydrogen-hydrogen H-bonding represents just such an interaction, and Radu Custelcean's work in this area amply demonstrated the idea. He established that loss of H2 from HHH-bonds in the solid state can lead to products distinct from those in fluid phases (melt or solution) and that in favorable cases, crystallinity can be maintained throughout the reaction despite some geometry change and the release of gas inside the crystals [45,47]. It is now clear that HHH-bonding can be used as a key element in crystal engineering. Because of their reliable structural preferences and compactness, HHH-bonds may serve as key elements in strategies aimed at synthesis of new covalent linkages in solid crystal lattices [52,54]. We have recently reviewed the chemistry of this interesting interaction [57], and presented our first efforts to assemble extended covalently bonded networks via the above HHH-bond-directed pathways.[60, 62].
The hydridic-to-protonic hydrogen bonding project, begun in the 1994 with high school student Mani Sharma, has benefited from the participation of several talented and hardworking high school students--Mani Sharma ('94), Charley Wilson ('95), Susanna Bass ('96), and Jennifer Hung ('97). Three of the four entered the Westinghouse Science Talent Search competition, and two (Sharma and Bass) were semifinalists, among their many awards. The X-ray structure of guanidinium borohydride, an interesting example of multipoint HHH-bonding, came out of Susanna Bass's studies in the summer of 1996. After Drs. Gatling and Custelcean moved on to new horizons, Simona Marincean, aided by such talented undergraduate researchers as Gates Cambridge Scholar Robin Stein, Melinda Baker, and Justin Roberts (all now attending top ten graduate schools in Chemistry), uncovered new segments of this unusual story. Simona, now Dr. Marincean, used ab initio methods to study the dynamics of proton transfer in the reaction of various acids with BH4 and AlH4 anions. [71] Together with Custelcean and Stein, she also reexamined the crystal structure of NH4H2PO2, a salt whose crystal structure, reported in 1934 before the "classical" hydrogen bond had even been recognized as a common structural theme. This compound's crystal structure had been analyzed in terms which implied hydridic-to-protonic hydrogen bonding, but though their insight turned out to be propetic, our modern structural reanalysis found no such bonding in this compound. [74]
Related Literature
45. Custelcean, R.; Jackson, J. E. "Topochemical Control of Covalent Bond Formation by Dihydrogen Bonding" J. Am. Chem. Soc. 1998, 120, 12935-12941
47. Custelcean, R.; Jackson, J. E. "Tuning Dihydrogen Bonds: Enhanced Solid State Reactivity in a Dihydrogen Bonded System with Exceptionally Short HH Contacts" Angew. Chem. Int. Ed. 1999, 38, 1661-1663.
50. Gatling, S. C.; Jackson, J. E. "Reactivity Control via Dihydrogen Bonding: Diastereoselection in Borohydride Reductions of α-Hydroxyketones" J. Am. Chem. Soc.1999, 121, 8655-8656.
52. Custelcean, R.; Jackson, J. E. "Topochemical Dihydrogen to Covalent Bonding Transformation in LiBH4.TEA: A Mechanistic Study" J. Am. Chem. Soc. 2000, 122, 5251-5257.
54. Custelcean, R.; Vlassa, M.; Jackson, J. E. "Toward Crystalline Covalent Solids: Crystal-to-Crystal Dihydrogen to Covalent Bonding Transformation on NaBH4.THEC" Angew. Chem. Int. Ed. 2000, 39, 3299-3302.
57. Custelcean, R.; Jackson, J. E. "Dihydrogen Bonding: Structures, Energetics, and Dynamics" Chem. Rev. 2001, 101, 1963-1980.
60. Custelcean, R.; Vlassa, M.; Jackson, J. E. "Supramolecular Synthesis with Dihydrogen Bonds: Assembly of Controlled Architectures from Polyhydroxyethylcyclen.NaBH4 Building Blocks" Chem. Eur. J. 2002, 8, 302-308.
62. Custelcean, R.; Jackson, J. E. "A Kinetic Study of a Topochemical Dihydrogen to Covalent Bonding Transformation" Thermochimica Acta, 2002, 388, 143-150. (article in pdf format).
71. Marincean, S.; Jackson, J. E. "Quest for IR-Pumped Reactions in Dihydrogen-Bonded Complexes" J. Phys. Chem. A 2004, 108, 5521-5526.
74. Marincean, S.; Custelcean, R.; Stein, R. S.; Jackson, J. E. "Structural Reinvestigation of Ammonium Hypophosphite: Was Dihydrogen Bonding Observed Long Ago?" Inorg. Chem., 2005, 44, 45-48.