Project Members
Tayeb Kakeshpour
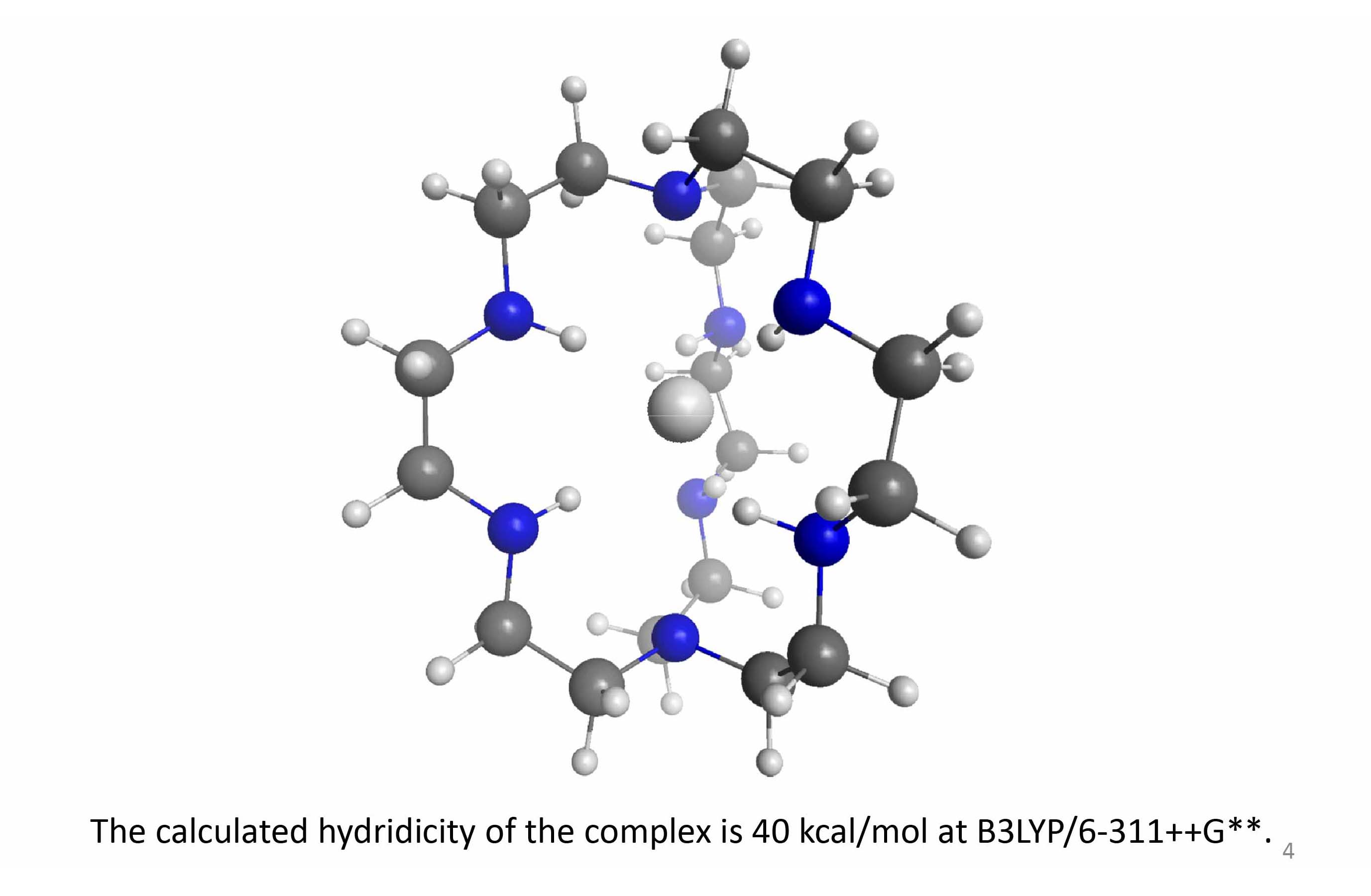
Perazacryptand as a Host for Hydride: Extreme Case of HHH-Bonding
Project Overview
Amine N-H bonds are good candidates for hydrogen bonding with basic anions. In general, the complexing capacity of a moiety X-H in a hydrogen bond X-H…Y- (X, Y = electronegative atoms or groups) increases with the acidity of X-H, or more precisely, with the stability of the X- anion. However, for basic Y- partners, proton transfer from the donor X-H to the acceptor Y- limits the range of X-H partners to stabilize the conjugate bases Y- of acids weaker than Y-H. Amine N-H bonds are, on one hand, polar enough to make hydrogen bonds, and on the other hand, non-acidic enough to survive even the presence of solvated electrons. They are thus suitable for stabilizing basic anions like fluoride, the solvated electron, and the hydride anion, as extreme examples.
In octazacryptand (IUPAC name: 1,4,7,10,13,16,21,24-octaazabicyclohexacosane), the crystal structure reveals that the four N-H groups point inward, creating a hydrogen bonding cavity which should readily bind small Lewis basic anions such as fluoride. In fact, the hexa-protonated octazacryptand has been shown to host fluoride and chloride, but not the larger bromide. In a direct competition, this binding selects fluoride over chloride, presumably due to the small cavity size in the relaxed octazacryptand, which appears to be nearly a perfect fit for fluoride. The larger goal of this project is the isolation of hydride anion inside the octazacryptand. However, the easier fluoride ion, which is thought to have an ionic radius very close to hydride, is chosen to be the prior binding target as a confirmation for hydride encapsulation potential. The hexa-protonated octazacryptand holds fluoride ion inside. Lehn et al have shown that hexa-, penta-, tetra-, and tri-protonated octazacryptand binds to fluoride in water with the stability constants of 1010, 108, 105, and 103, respectively. They even grew single crystals of the hexa-protonated ligand complexing fluoride at the center, which is definitive proof. This crystal structure is an evidence that proves the desired conformer of the neutral ligand is physically and energetically available. Their crystallographic data show F-....N+ distances ranging from 2.76 to 2.88 Å. Based on gas phase EDF2/6-311++G** calculations, the neutral ligand can readily fit fluoride inside, and the binding energy is calculated to be 60 kcal/mol.
Related Literature
Coming Soon