Project Members
Prof. Christopher Saffron (Collaborator)
Gracielou Klinger
Benjamin Appiagyei
Yuting Zhou
Electrocatalytic Upgrading of Fast Pyrolysis Oil to Chemical and Fuel
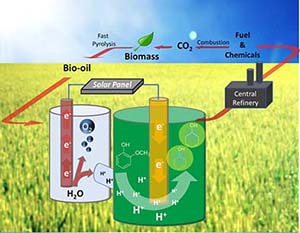
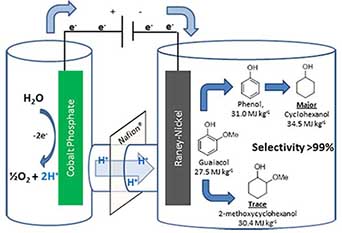
Project Overview
Considering impending petroleum shortages, the environmental costs of using fossil-based fuels, the limitations of the hydrogen-based approach for transport, and the irreplaceable nature and ever growing demand for hydrocarbons, there is a need to shift hydrocarbon production in the US, and ultimately worldwide, to a renewable source: Biomass. Compared to production of starch-based ethanol and biodiesel, large-scale pyrolysis-based conversion of biomass into liquid fuels is still considered to be an emerging field. Flash pyrolysis (rapid heating in the absence of oxygen) breaks down the plant material (cellulose and other tissue components) into small-molecule species in three states: flammable gases, bio-liquids, and solid char. While the flammable gas and solid char can be used as fuels without pretreatment, the liquid (aka bio-oil) is the major fraction (ca. 60-70%) and represents the starting point for potential liquid fuels production. The specific composition of bio-oil varies based on the feedstock, but the main component classes are shared in common. Unfortunately, bio-oil cannot be used without pre-treatment for several reasons: a) low energy density: like biomass, bio-oil has ca. 1/3 the energy content of hydrocarbons (ca. 15 vs. ca. 45 MJ/kg) b) high acidity: bio-oil contains ca. 20% of acetic acid, along with phenolic species; this mixture corrodes steel aggressively at elevated temperature4 c) Reactivity and viscosity: bio-oil tends to polymerize via condensation of its high content of reactive oxygenates such as aldehydes and ketones; d) water content: bio-oil is an emulsion which makes it incompatible with storage and transport over extended periods. Hence, bio-oil must be pretreated or upgraded before it can even be considered as a crude fuel.
To increase the energy density of bio-oil, and to minimize condensation reactions, it is crucial to deoxygenate the organic compounds. However, direct deoxygenation has proven to be challenging under mild conditions: general net hydrogenation and deoxygenation processes are needed. These effectively incorporate the heat of combustion of H2 while lowering the mass of substrate compounds, raising the net energy density of the biomass fast pyrolysis oil
Related Literature
Lam, C. H.; Lowe, C. B.; Li, Z.; Longe, K. N.; Rayburn, J. T.; Caldwell, M. A.; Houdek, C. E.; Maguire, J. B.; Saffron, C. M.; Miller, D. J.; Jackson, J. E. "Electrocatalytic Upgrading of Model Lignin Monomers with Earth Abundant Metal Electrodes" Green Chem. 2015, 17, 601-609.
Li, Z.; Kelkar, S.; Raycraft, L.; Garedew, M.; Jackson, J. E.; Miller, D. J.; Saffron, C. M. "A Mild Approach for Bio-oil Stabilization and Upgrading: Electrocatalytic Hydrogenation Using Ruthenium Supported on Activated Carbon Cloth" Green Chem. 2014, 16, 844-852.
Li, Z.; Garedew, M.; Lam, C. H.; Jackson, J. E.; Miller, D. J.; Saffron, C. M. "Mild Electrocatalytic Hydrogenation and Hydrodeoxygenation of Bio-oil Derived Phenolic Compounds using Ruthenium Supported on Activated Carbon Cloth" Green Chem. 2012, 14, 2540-2549.
Li, Z.; Kelkar, S.; Lam, C. H.; Luczek, K.; Jackson, J. E.; Miller, D. J.; Saffron, C. M. "Aqueous Electrocatalytic Hydrogenation of Furfural Using a Sacrificial Anode" Electrochim. Acta 2012, 64, 87-93.