|
The
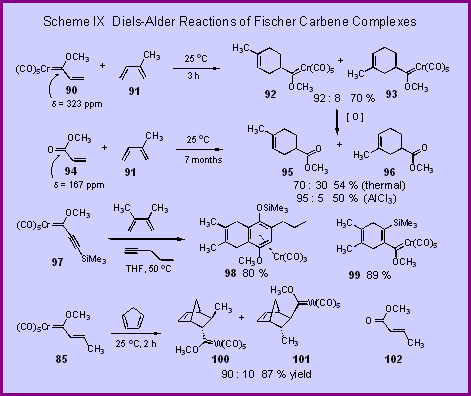
second is shown in Scheme IX and is the comparison of the
chemical shift of the carbene carbon of the vinyl complex 90 versus that of corresponding ester 94. The carbene carbon
is at d = 323 ppm which is much father downfield than the ester 94
(d = 167). This
data suggests that the carbene complex should have a much
greater electron withdrawing effect on the double-bond than the
ester group and thus that complex 90
should be a much stronger dienophile than the ester 94.
Our expectations were realized when we found that the
complex 90 would
react with isoprene 21,000 times faster than the ester 94 [1].[In
addition to the increased rate, the reaction of the complex 90
is also more regioselective than the ester 94
(92 : 8 vs. 70 : 30). The
increased rate and increased regioselectivity observed for the
reaction of the complex 90
is comparable to the increase in rate (74,000) and
regioselectivity (95 : 5) observed when the reaction of methyl
acrylate and isoprene is catalyzed by aluminum chloride. Since
the cycloadduct carbene complexes 92
and 93 |
|
|
can
be efficiently converted to the esters 95 and 96 by a
variety of mild oxidizing agents,  -unsaturated carbene complexes can serve as surrogates for esters in the
Diels-Alder reaction when the use of Lewis acid is best avoided. The Diels-Alder reaction of Fischer carbene complexes can also be
extended to acetylenic complexes. The reaction of complex 97
with 2,3-dimethylbutadiene gives the cycloadduct 99 in 89 % yield at 50 oC in 4 hours [2].
This is an -unsaturated carbene complexes can serve as surrogates for esters in the
Diels-Alder reaction when the use of Lewis acid is best avoided. The Diels-Alder reaction of Fischer carbene complexes can also be
extended to acetylenic complexes. The reaction of complex 97
with 2,3-dimethylbutadiene gives the cycloadduct 99 in 89 % yield at 50 oC in 4 hours [2].
This is an  -unsaturated complex, which should be useful
in the benzannulation reaction.
In fact, the reaction of 99
with 1-pentyne gives the benzannulation product 98
in 97 % yield. This
overall transformation can be carried out in a one-pot process
with all three reagents together since the carbene complex will
selectively react with the diene over the alkyne.
The one-pot reaction gives the benzannulated product 98
in 80 % yield. This
benzannulation gives a benzene ring rather than a
cyclohexadienone since a silyl group at either R1 or
R2 in intermediate 16
(Scheme
V) will tautomerize via 1,3-migration to oxygen which
conveniently gives a protected phenol chromium tricarbonyl
complexes which, as discussed above (Scheme
IV), are stable to air and can be isolated.
-unsaturated complex, which should be useful
in the benzannulation reaction.
In fact, the reaction of 99
with 1-pentyne gives the benzannulation product 98
in 97 % yield. This
overall transformation can be carried out in a one-pot process
with all three reagents together since the carbene complex will
selectively react with the diene over the alkyne.
The one-pot reaction gives the benzannulated product 98
in 80 % yield. This
benzannulation gives a benzene ring rather than a
cyclohexadienone since a silyl group at either R1 or
R2 in intermediate 16
(Scheme
V) will tautomerize via 1,3-migration to oxygen which
conveniently gives a protected phenol chromium tricarbonyl
complexes which, as discussed above (Scheme
IV), are stable to air and can be isolated.
|
|
As
with normal Diels-Alder reactions, the Diels-Alder
reactions of alkoxy carbene complex are usually endo
selective. The reaction of complex 85
with cyclopentadiene gives a 90 : 10 ratio of the endo
and exo cycloadducts 100
and 101. This is to be compared
with the thermal reaction of the corresponding ester 102 with cyclopentadiene which gives a 54 : 46 ratio of the endo to
exo cycloadducts. The
endo selectivity of 102
can be improved to 93 : 7 if its reaction with
cyclopentadiene is catalyzed by aluminum chloride.
The endo stereoselectivity observed from the
reaction of Fischer
carbene complexes is usually comparable to that seen
from Lewis acid catalyzed reactions of the corresponding
esters. Since
carbene complexes are much more compatible with
sensitive organic functional groups than most Lewis
acids, Fischer carbene complexes can serve as useful
synthons for esters in Diels-Alder reactions. |
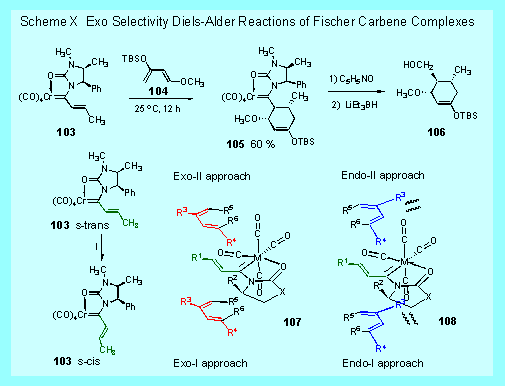
One
of the limitations of the Diels-Adler reaction is, of course,
that it is endo selective for most dienophiles and dienes.
We have found that imidazolidinone carbene complexes of
the type 103 are exo selective [3].[The
reason for this is thought be a consequence of the preference
the s-cis conformation over the s-trans conformation of 103.
The Diels-Alder reaction from the s-cis conformation
results in close contacts between the substituent R3
on the diene and a cis carbon monoxide ligand on the metal that
are present in the endo transition states but not in the exo
transition states. In
addition, the chiral complex 103
results in complete facial selectivity in the addition to the
diene leading to a single diastereomer of 105
and, after removal of the chiral auxiliary, a single enantiomer
of the exo cycloadduct 106. Since the Diels-Alder
reactions of imidazolidinone complexes can be highly exo
selective it is anticipated that these complexes would have
unique applications in organic synthesis that are not possible
by traditional Diels-Alder strategies.
[1]
Wulff, W. D.; Bauta, W. E.;
Kaesler, R. W.; Lankford, P. J.; Miller, R. A.; Murray, C.
K.; Yang, D. C., J.
Am. Chem. Soc., 1990,
112, 3642.
[2]
Wulff, W. D.; Yang, D. C.,
J. Am. Chem. Soc.,
1984, 106, 7565.
[3]
Powers, T. S.; Jiang, W.;
Su, J.; Wulff, W. D., J.
Am. Chem. Soc., 1997,
119, 6438.
|

