|
Asymmetric Catalysis in Organic Synthesis |
|
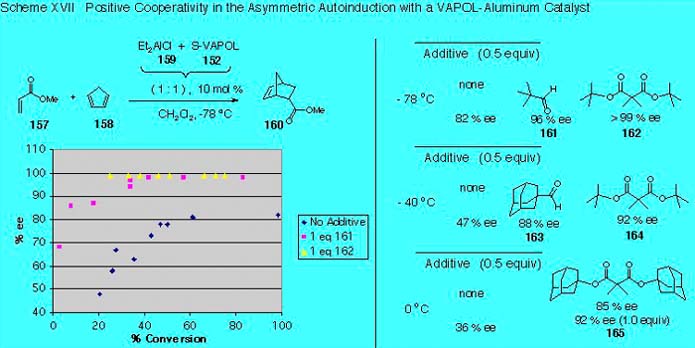
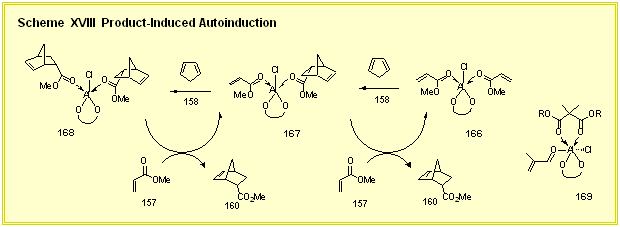
II. Catalytic Asymmetric Diels-Alder Reactions We have previously published that Diels-Alder reactions mediated by a catalyst prepared from the VAPOL ligand and diethyaluminum chloride occurs with an autoinduction [1]. An auto-induction is seen with both aldehydes and esters but the reactions with aldehydes are too fast to conveniently allow for following reactions over time. In addition we have seen in our screening of several substrates that the effect is much bigger for esters. The auto-induction for the reaction of methyl acrylate and cyclopentadiene is shown in Scheme XVII. The first data point is at about 20 percent conversion and reveals that the induction of the product is 47 % ee. During the course of the reaction this induction rises and at the end of the reaction the product is 82 % ee. The basic tenant of the mechanistic hypothesis that was developed early in our studies of this phenomenon was that more than one carbonyl compound was coordinated to the aluminum. One possible scenario is shown in Scheme XVIII where the active catalyst species 166 and 167 involve pentacoordinate aluminum. Assuming carbonyl exchange is rapid, the autoinduction could be explained if it is hypothesized that the catalyst species 167, coordinated by one dienophile and one product molecule, gives a higher induction in its Diels-Alder reaction than that of species 166 which contains two coordinated dienophiles. If this mechanistic interpretation were correct, then at the beginning of the reaction species 166 would be more abundant and the selectivity would be lower than during the latter parts of the reaction where species 167 would be more abundant. Consistent with the mechanism in Scheme XVIII is the observation that if optically pure product is added at the beginning of the reaction, the optical purity of the product at the end of the reaction is increased. We have also found that simple carbonyl and dicarbonyl compounds can serve as product mimics and enhance the enantioselection as illustrated by the data in Scheme XVII. The effectiveness of the product mimics pivaldehyde 161 and di-t-butyl malonate 162 in the auto-induction of the Diels-Alder reaction of methyl acrylate with cyclopentadiene on the induction was monitored as a function of the % conversion at -78 oC (Scheme XVII). The reaction in the presence of 1.0 equivalent of pivaldehyde 161 begins at a much higher induction and rises during the course of the reaction to give a much higher optical purity in the product than in the absence of any additive (98 % ee versus 82 % ee). The final induction of 98 % means that 1 % of the enantiomer of 160 (actually 1.2 %) is present at the end of the reaction. Analysis of the curve for pivaldehyde 161 reveals that all of the minor enantiomer is essentially formed in the first 15 percent of the reaction and that in the remaining 85 % of the reaction the catalyst is functioning at levels of above 99.5 % ee. Even more dramatic is the plot with di-t-butyl malonate. Within the limits of detection, the cooperativity of 1.0 equivalent of 2,2-dimethyl-di-t-butyl malonate is perfect as judged by the fact that the product is optically pure (≥99 % ee) at the first data point taken (24 % conversion) and remains so during the course of the reaction. The malonate-mediated reaction may involve the hexacordinate aluminum species 169 assuming that the chloride does not ionize to produce an aluminum cation.
The surprise from this study is that the selectivity for aluminum catalysts would be sensitive to the addition of coordinating ligands. For aluminum the most common coordination number is four. Higher coordination numbers for aluminum are rarely invoked to explain the outcome of stereoselective reactions. Most organoaluminum compounds are bridged dimeric species that are usually four coordinate. However, there are examples of five and six coordinate organoaluminum species that are also mononuclear and which have been characterized by X-ray diffraction. [1] Heller, D. P.; Goldberg, D. R.; Wulff, W. D., J. Am. Chem. Soc., 1997, 119, 10551.
|
Asymmetric Catalysis I. Ligand Design and Synthesis II. Asymmetric Diels-Alder Reaction
II. Cyclohexadienone Annulation III. Tautomer Arrested Annulation
Synthesis of Natural Products and Pharmaceuticals
|