|
Fischer Carbene Complexes in Organic Synthesis |
|
|
Carbene Complexes Introduction II. Cyclohexadienone Annulation III. Tautomer Arrested Annulation VIII. Macrocycles
I. Ligand Design and Synthesis II. Asymmetric Diels-Alder Reaction III. Imino Aldol Reaction
Synthesis of Natural Products and Pharmaceuticals
|
Our research group has had a long-standing interest in the chemistry of Fischer carbene complexes especially in their applications in organic synthesis. The attraction had been two-fold: the unique transformations that the reactions of these complexes offer and the great diversity of the different types of reactions that they undergo. The reactions of Fischer carbene complexes that have been examined for utility in synthetic organic chemistry include the benzannulation of unsaturated complexes with alkynes, the cyclohexadienone annulation, the two-alkyne annulation, cyclopropanations, [2 + 2] cycloadditions, [3 + 2] cycloadditions, [4 + 1] cycloadditions, Ene reactions, Diels-Alder reaction, Michael additions, aldol reactions, alkylations, furan formation, indene formation, cyclobutanone formation, beta-lactam formation, gamma-lactone formation, gamma-lactam formation, cyclopentenone formation, cyclopentenedione formation, pyrrole formation, 3-hydroxypyridine formation, C-H insertion reactions, Moore-Type cyclizations in indole formation, tautomer arrested annulations, 1,2-addition reactions, and macrocyclizations. These are some of the reactions that our research group has been involved with and many other reactions have been examined by a number of other research groups. Reviews concerning the applications of Fischer carbene complexes in organic synthesis have been published by us [1] and by others[[2]. As a result of the singularity and of the great breadth of the reactions of this single class of reagents, a good case can be made that Fischer carbene complexes are the most versatile class of organometallics reagents for applications in synthetic organic chemistry.
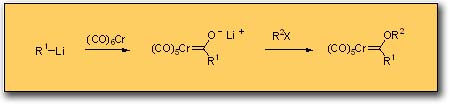
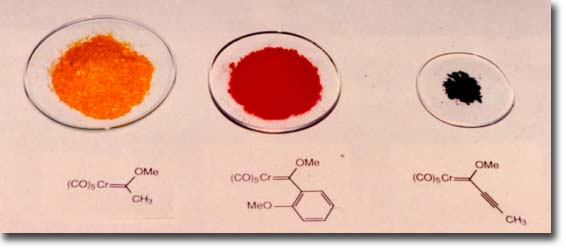
One of the attractive features of Fischer carbene complexes that drew us to their study is their ease of preparation and their stability. Although a number of methods have been developed for their synthesis, the most widely used method is still that originally reported by Fischer which involves the addition of an organo-lithium to chromium carbonyl followed by alkylation on oxygen. This preparation is simple and rapid and can be performed on opened ended scale since most complexes are solids and can thus be purified by crystallization. The complexes are stable to air and water and to dilute acids and bases. The workup typically involves washing the organic solution of the carbene complex in a separatory funnel with dilute aqueous base. Despite the high dipole moment of these complexes (~ 4 - 5 Debye), most complexes can be purified by chromatography on silica gel with hexane as eluent and are usually the first compounds to elute. Identification of the fractions from the column containing the carbene complex can simply be done by eye on the basis of their color. The colors of complexes bearing alkoxy groups as the heteroatom-stabilizing group tend to correlate with the hybridzation of the carbon substituent of the carbene carbon. Those with sp3 carbons usually are yellow, those with sp2 carbons are normally red and those with sp hybridized carbon substituents are invariably an intense purple/black color.
[1] a) Wulff, W. D. in Advances in Metal-Organic Chemistry; Liebeskind, L. S., Ed.; JAI Press Inc.: Greenwich, Conn, 1989; Vol. 1. b) Wulff, W. D., in "Comprehensive Organic Synthesis", Trost, B. M.; Fleming, I., Eds., Pergamon Press, 1990, Vol 5. c) Wulff, W. D. in Comprehensive Organometallic Chemistry II, Abel, E.W.; Stone, R.G.A.; Wilkinson, G., Eds.; Pergamon Press, 1995, Vol. 12, 469. d) Wulff, W. D., Organometallics, 1998, 17, 3116. e) Waters, M. L.; Wulff, W. D., Organic Reactions, in press. [2]
a) Hegedus, L. S., Tetrahedron,
1997, 53, 4105.
b) de Meijer, A.; Schirmer, H.; Duetsch, M., Angew.
Chem. Int. Ed. Engl., 2000,
39, 3964. c) Dötz,
K. H.; Tomuschatt, P., Chem. Soc.
Rev., 1999, 28 187. d) Herndon, J.
W., Coord. Chem. Rev., 1999,
181, 177. e)
“Metal Carbenes in Organic Synthesis”, Dörwald, F. Z.,
Wiley-VCH, 1999. f) Bernasconi, C. F., Chem. Soc. Rev., 1997,
26, 299. g) M. Doyle in "Comprehensive Organometallic Chemistry
II", E. W. Abel; F. G. A. Stone; G. Wilkinson, Eds., Pergamon
Press, 1995, Vol 12. h) Harvey, D. F.; Sigano, D. M., Chem.
Rev., 1996, 96, 271. |
|
|