|
Although
the cyclohexadienone annulation is successful for indole carbene
complexes (Scheme V), cyclohexadienones canít be obtained from the
reaction of 2,6-disubstituted phenyl complexes.
The reaction of these complexes occur without the incorporation
of a carbon monoxide ligand of the carbene complex to give indene
products of the type 65 which result from a 1,5-sigmatropic shift of the one of the
ortho-substituents in 63 [1].[
A specific example is the formation of the indene 67
from the reaction of diisopropyl acetylene and the 2,6-dimethylphenyl
carbene complex 66.
It was thought that the driving force for the 1,5-sigmatropic
shift of the methyl group arose from the
aromaticity
that is regained in the benzene ring.
|
|
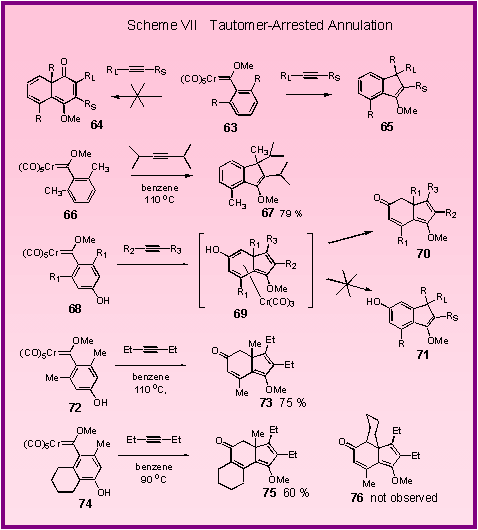 |
It
was thus proposed that the 1,5-sigmatropic shift of methyl might
be prevented if a tautomerization of an enol of the type 69
to give the ketone 70
were to occur. This
in fact proved to be possible [2].[The
reaction of the hydroxy-substituted carbene complex 72
with 3-hexyne gave the hydroindenone 73
in 75 % yield revealing that the tautomerization of the enol is
faster than the 1,5-sigmatropic shift of methyl.
The reaction is also regioselective as illustrated by the
reaction of complex 74
with 3-hexyne, which gave the product resulting from
cyclizations to the methyl position rather than to the methylene
position. The
simplicity of this reaction for the construction of highly
functionalized perhydroindenones makes it an attractive
candidate for applications in total synthesis.
[1]
Quinn, J. F.; Bos, M. E.; Wulff, W. D., Org.
Lett., 1999, 1, 161.
[2]
Bos, M. E.; Wulff, W. D.; Wilson, K. J., J. Chem. Soc.,
Chem. Commun., 1996,
1863
|