|
|
|
|
methodology & mechanism Organotin Chemistry Organosilane ChemistryOrganoborane Chemistry total synthesis Please click on the links above for a more detailed research description |
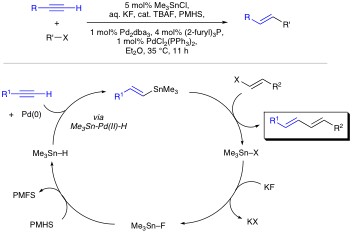
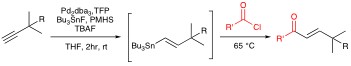
Organotin Chemistry Organotin compounds are versatile reagents in organic chemistry. Unfortunately, their utilization is not without problems. Organotins tend to be relatively expensive, unstable, and toxic. Furthermore, tin byproducts are not always easy to separate and represent an ever increasing disposal problem. Yet because of their synthetic utility, the use of organotins remain common place in academic and even industrial syntheses. One aim of our research program is to minimize the toxicity and environmental hazards associated with organotins. In this regard, much of our focus has been on the development of new chemical transformations in which the tin specie is traditionally not a reagent , but rather a reactant. In particular we have been exploring "Tin-Lite" options to reactions such as the Stille cross-coupling. Stille reactions typically involve the palladium catalyzed cross coupling of organostannanes with a variety of organic electrophiles. Owing to its versatility and functional group compatibility, the Stille reaction has long been a popular method for the construction of sp2–sp2 carbon–carbon bonds via sigma bond formation. That said, Stille reactions typically demand the management of stoichiometric amounts of tin before, during, and after the cross-couplings. This is unattractive because of the aforementioned troubles associated with the cost and toxicity of organotins. To address the Stille reaction’s “tin problem” we seek a Stille reaction that is catalytic in tin. Our current generation of such a process involves a tandem Pd-catalyzed hydrostannation/cross-coupling protocol. Polymethylhydrosiloxane (PMHS) made hypercoordinate by KF(aq.) allows Me3SnH to be recycled during the Pd(0)-catalyzed hydrostannation/Stille cascade. Starting with a variety of alkynes, in situ vinyltin formation is followed by Stille reaction with aryl, styryl, benzyl, or vinyl electrophiles present in the reaction mixture. Both inter- and intramolecular versions of the process are possible with tin loads of approximately 6 mole %.
Simultaneously we developed one-pot Pd-catalyzed hydrostannation/Stille coupling sequences that recycle stoichiometric amounts of tin between steps. A recent description of this chemistry was recognized as a "top-50 most downloaded" article from the Journal of Organometallic Chemistry on ScienceDirect during 2006.
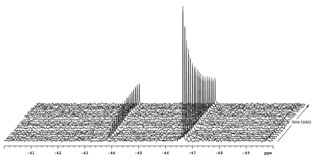
To take this chemistry to the next level we need to learn more about the reaction mechanism. Our preliminary findings suggest 119Sn-NMR to be an excellent way to analyze the kinetics these reactions (Figure 2).
Selected organostannane and cross-coupling related publications: A One-Pot Allylation-Hydrostannation Sequence with Recycling of the Intermediate Tin Waste” Ghosh, B.; Amado-Sierra, M. R. I.; Holmes, D.; Maleczka, R. E.; Jr. Org. Lett. 2014, 16, 2318–2321. “Non-Pd Transition Metal Catalyzed Hydrostannations: Bu3SnF/PMHS as a Tin Hydride Source” Ghosh, B.; Maleczka, R. E., Jr. Tetrahedron 2013, 69, 4000–4008. “Ni,
Co, & Mo-metal-catalyzed alkynes
hydrostannations using Bu3SnCl/PMHS/KF/18-crown-6
as an in
situ Bu3SnH source” Ghosh, B.; Maleczka, R.
E., Jr. Tetrahedron
Lett. 2011,
52, 5285–5287. “Reactions of
Vinyltributylgermanes and Aryl Halides
under Heck Conditions” Torres, N. M.; Lavis, J. M.;
Maleczka, R. E.,
Jr. Tetrahedron Lett.
2009, 50, 4407–4410. “Aryl–Aryl Cross-Couplings that
Avoid the Preparation
of Haloaromatics” Norberg, A. M.; Sanchez, L.; Maleczka,
R. E., Jr. Curr.
Opin. Drug Discov. Dev. 2008, 11, 853–869. “One-Pot Pd-Catalyzed Hydrostannation/Stille Reaction with Acid Chlorides as the Electrophiles” Lee, K.; Gallagher, W. P.; Toskey, E. A.; Chong, W.; Maleczka, R. E., Jr. J. Organomet. Chem. 2006, 691, 1462–1465. “Stille Reactions Catalytic in Tin: A “Sn-F” Route for Intermolecular and Intramolecular Couplings” Gallagher, W. P.; Maleczka, R. E., Jr. J. Org. Chem. 2005, 70, 841–846. “Stille Couplings Catalytic in Tin: A “Sn–F” Approach” Maleczka, R. E., Jr.;Gallagher, W. P. Org. Lett. 2001, 3, 4173–4176. “The Regiochemical Influence of Oxo-Substitution in Palladium Mediated Hydrostannations of 1-Alkynes” Rice, M. B.; Whitehead, S. L.; Horvath, C. M.; Muchnij, J. A.; Maleczka, R. E., Jr. Synthesis 2001, 1495–1504. “Stille Couplings Catalytic in Tin — The 'Sn–O' Approach” Gallagher, W. P.; Terstiege, I.; Maleczka, R. E., Jr. J. Am. Chem. Soc. 2001, 123, 3194–3204. |