
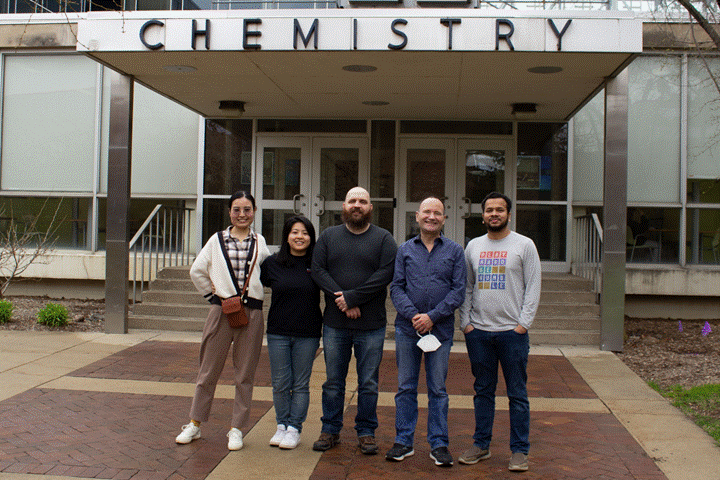
Group
Picture (April 2022)
Starring:
David Weliky as the faculty member –
Weliky CV
Yijin Zhang as a graduate research associate
Md Rokonujjaman as a graduate research associate
Noel Chau as a graduate research associate
Forkan Saroar as a graduate research associate
Tahmina Khatun as a graduate research associate
FORMER
GROUP MEMBERS
Robert Wolfe (Ph. D. 2022, Scientist at Michigan State University)
Ahinsa Ranaweera (Ph. D. 2019, Senior Lecturer at Rajarata University
of Sri Lanka)
Shuang Liang (Ph. D. 2017, Scientist at Alcon)
Lihui Jia (Ph. D. 2017, Scientist at Alcon)
Ujjayini Ghosh (Ph. D. 2016, Faculty at University of California,
San Francisco)
Punsisi Ratnayake (Ph. D. 2015) Scientist at Katahdin Analytical
Services
Li Xie (Ph. D. 2014, Scientist at Michigan State University)
Koyeli Banerjee (Ph. D. 2014, Health Scientist Administrator at
ARHQ/DHHS)
Scott Schmick (Ph. D. 2013, Scientist at 3M)
Kelly Sackett (Scientist at Pfizer)
Erica Vogel (Ph. D. 2012)
Owner of Be Like Missy boutique
Matthew Nethercott (Ph. D. 2012, Scientist at Kansas Analytical
Services)
Yan Sun (Ph. D. 2009, Instructor at Binghamton University)
Wei Qiang (Ph. D. 2009, Professor at Binghamton University)
Jaime Curtis-Fisk (Ph. D. 2009, Scientist at Dow Chemical)
Matthew Gave (Ph. D. 2008, Scientist at SK Siltron
CSS)
Zhaoxiong
Zheng (Ph. D. 2007, Scientist at
Shell Oil)
Michele Bodner Mailhot (Ph.
D. 2006, Scientist at Colorado State University)
Paul Parkanzky (Ph. D. 2006, Scientist at Amway)
Rong Yang (Ph. D. 2005, Scientist at National
Institutes of Health)
Christian Canlas (Ph. D. 2004, Scientist
at the King Abdullah University of Science & Technology)
Jun Yang (Ph. D. 2003,
Scientist at Lerner Research Institute, Cleveland Clinic)
Bhagyashree Khunte (M.
S. 2001, Scientist at Pfizer Corporation)
Contact Information
Dr. Weliky
telephone: 517-353-1177
Graduate Student/Post-doc
telephone: 517-353-1178
Fax: 517-353-1793
Research
The Weliky group specializes in solid-state NMR
spectroscopy with particular application to biological systems. Solid-state NMR
is a powerful approach to structural and dynamical measurements on biological
molecules and is particularly useful for studies in non-crystalline
environments such as membranes and bacterial inclusion bodies. We spend about
80% of our effort on applications and 20% of our effort on new methods
development. Our research includes physical, analytical, organic, and
biological chemistries.
Applications
We are currently studying the structure of
the membrane-associated HIV gp41 envelope protein. One project focuses on the
fusion peptide region of gp41, which is required for viral/host cell membrane
fusion. We are studying this peptide as well as the whole gp41 protein in the
most biologically relevant membrane environment. Our goal is to better
understand the structural basis of membrane fusion which should be useful for
designing new AIDS therapeutics. We also have a related project on the
influenza virus fusion protein.
A second area is the structure of recombinant
proteins in bacterial inclusion bodies. Academic and pharmaceutical
laboratories commonly produce large quantities of a protein by introducing the
gene for this protein into E. coli
bacteria and having the bacteria make the protein. A common problem is
sequestration of the foreign protein in non-crystalline solids termed inclusion
bodies. Better understanding of the protein structure in inclusion bodies
should lead to better methods of obtaining useful protein from inclusion
bodies.
Equipment
NMR
We have access to two 400 MHz Bruker Neo
spectrometers, one Varian Chemagnetics Infinity Plus
spectrometer, and one 800 MHz Bruker Neo spectrometer. In addition, we have a
variety of magic angle spinning and static probes.
Biochemical
We have an incubator shaker, refrigerated
centrifuges, preparative HPLC, FPLC, uv-vis
spectrophotometer, and lyophilizer. We also have a
nitrogen/vacuum line for organic derivatization of amino acids.
Publications (in pdf format)
1.
Y. Zhang, U. Ghosh, L. Xie, D. Holmes, K. P.
Severin, and D. P. Weliky, “Lipid Acyl Chain Protrusion Induced by the Influenza
Virus Hemagglutinin Fusion Peptide Detected by NMR Paramagnetic Relaxation Enhancement”,
Biophysical Chemistry, 299, 107028 (2023).
2.
Md. Rokonujjaman, A. Sahyouni, R. Wolfe, L.
Jia, U. Ghosh, and D. P. Weliky, “A
Large HIV gp41 Construct with Trimer-of-Hairpins Structure Exhibits V2E
Mutation-Dominant Attenuation of Vesicle Fusion and Helicity very similar to V2E
attenuation of HIV Fusion and Infection and Supports: (1) Hairpin Stabilization
of Membrane Apposition with Larger Distance for V2E; and (2) V2E dominance by
an Antiparallel b
sheet with Interleaved Fusion Peptide Strands from Two gp41 Trimers”, Biophysical Chemistry, 203,
106933 (2023).
3.
U. Ghosh and D. P. Weliky, “Rapid 2H
NMR Transverse Relaxation of Perdeuterated Lipid Acyl Chains of Membrane with
Bound Viral Fusion Peptide Supports Large-Amplitude Motions of These Chains
That Can Catalyze Membrane Fusion”, Biochemistry,
60, 2637-2651 (2021).
4.
V. Jain, T. Shelby, T. Patel, E. Mekhedov,
J. D. Petersen, J. Zimmerberg, A. Ranaweera, D. P.
Weliky, P. Dandawate, S. Anant, S. Sulthana, Y.
Vasquez, T. Banerjee, and S. Santra, “A Bimodal Nanosensor
for Probing Influenza Fusion Protein Activity Using Magnetic Relaxation”, ACS Sensors, 6, 1899-1909 (2021).
5.
U. Ghosh and D. P. Weliky, “2H Nuclear
Magnetic Resonance Spectroscopy Supports Larger Amplitude Fast Motion and
Interference with Lipid Chain Ordering for Membrane that Contains β Sheet
Human Immunodeficiency Virus gp41 Fusion Peptide or Helical Hairpin Influenza
Virus Hemagglutinin Fusion Peptide at Fusogenic pH”, Biochimica et Biophysica
Acta- Biomembranes (2020) 1862,
183404.
6.
A. Ranaweera, P. U. Ratnayake,
E. A. P. Ekanayaka, R. Declercq, and D. P. Weliky,
“Hydrogen−Deuterium Exchange Supports Independent Membrane-Interfacial
Fusion Peptide and Transmembrane Domains in Subunit 2 of Influenza Virus
Hemagglutinin Protein, a Structured and Aqueous-Protected Connection between
the Fusion Peptide and Soluble Ectodomain, and the Importance of Membrane
Apposition by the Trimer-of-Hairpins Structure”, Biochemistry (2019) 58,
2432-2466.
7.
A. Ranaweera, P. U. Ratnayake, and D. P.
Weliky, “The Stabilities of the Soluble Ectodomain and Fusion Peptide Hairpins
of the Influenza Virus Hemagglutinin Subunit II Protein Are Positively
Correlated with Membrane Fusion”, Biochemistry,
57, 5480-5493 (2018).
8.
S. Liang, P. U. Ratnayake, C. Keinath, L.
Jia, R. Wolfe, A. Ranaweera, and D. P. Weliky, “Efficient Fusion at Neutral pH
by Human Immunodeficiency Virus gp41 Trimers Containing the Fusion Peptide and
Transmembrane Domains”, Biochemistry,
57, 1219-1235 (2018).
9.
P. N. Grenga, M. J.
Nethercott, A. E. Mateo, M. Paternaude, T. Hoare, D.
P. Weliky, and R. Priefer, “Thermal and Spectral
Analysis of Novel Amide-Tethered Polymers from Poly(allylamine)”, Australian Journal of Chemistry, 69, 458-466 (2016).
10.
P. U. Ratnayake, E. A. Prabodha Ekanayaka, S. S. Komanduru, and
D. P. Weliky, “Full-Length Trimeric Influenza Virus Hemagglutinin II Membrane
Fusion Protein and Shorter Constructs Lacking the Fusion Peptide or
Transmembrane Domain: Hyperthermostability of the
Full-Length Protein and the Soluble Ectodomain and Fusion Peptide Make
Significant Contributions to Fusion of Membrane Vesicles”, Protein Expression and Purification, 117, 6-16 (2016).
11.
U. Ghosh, L. Xie, L. Jia, S. Liang, and D. P.
Weliky, “Closed and Semiclosed Interhelical
Structures in Membrane vs Closed and Open Structures in Detergent for the
Influenza Virus Hemagglutinin Fusion Peptide and Correlation of Hydrophobic
Surface Area with Fusion Catalysis”, Journal
of the American Chemical Society, 137,
7548-7551 (2015).
12.
D. P. Weliky, “A New Understanding of
Antibiotic Action via Solid-State NMR of Cells with Uniform Isotopic Labeling”,
Biophysical Journal, 108, 1314 (2015).
13.
L. Jia, S. Liang, K. Sackett, L. Xie, U. Ghosh, and D.
P. Weliky, “REDOR Solid-State NMR as a Probe of the Membrane Locations of
Membrane-Associated Peptides and Proteins”, Journal
of Magnetic Resonance, 253,
154-165 (2015).
14.
L. Xie, L. Jia, S. Liang, and D. P. Weliky, “Multiple
Locations of Peptides in the Hydrocarbon Core of Gel-Phase Membranes Revealed
by Peptide 13C to Lipid 2H Rotational-Echo Double
Resonance Solid-State Nuclear Magnetic Resonance”, Biochemistry, 54,
677-684 (2015) – highlighted on Biochemistry
website.
15.
P. Ratnayake, K. Sackett, M. J. Nethercott, and
D. P. Weliky, “pH-Dependent Vesicle Fusion Induced by the Ectodomain of the
Human Immunodeficiency Virus Membrane Fusion Protein gp41: Two Kinetically
Distinct Processes and Fully-Membrane-Associated gp41 with Predominant β
sheet Fusion Peptide Conformation”, Biochimica et Biophysica Acta, 1848,
289-298 (2015).
16.
L. Cegelski and D. P. Weliky, “NMR Spectroscopy
for Atomistic Views of Biomembranes and Cell Surfaces”, Biochimica et Biophysica Acta, 1848, 201-202 (2015).
17.
K. Banerjee and D. P. Weliky, “Folded Monomers
and Hexamers of the Ectodomain of the HIV gp41 Membrane Fusion Protein:
Potential Roles in Fusion and Synergy Between the Fusion Peptide, Hairpin, and
Membrane-Proximal External Region”, Biochemistry,
53, 7184-7198 (2014).
18.
K. Sackett, M. J. Nethercott, Z.
Zheng, and D. P. Weliky, “Solid-State NMR Spectroscopy of the HIV gp41 Membrane
Fusion Protein Supports Intermolecular Antiparallel b Sheet Fusion Peptide
Structure in the Final Six-Helix Bundle State”, Journal of Molecular Biology, 426,
1077-1094 (2014).
19.
C. M. Gabrys, W. Qiang, Y. Sun, L. Xie, S. D.
Schmick, and D. P. Weliky, “Solid-State Nuclear Magnetic Resonance Measurements
of HIV Fusion Peptide to Lipid 31P Proximities Support Similar
Partially Inserted Membrane Locations of the a Helical and b
Sheet Peptide Structures”, Journal of
Physical Chemistry A, 117,
9848-9859 (2013).
20.
E. P. Vogel and D. P. Weliky, “Quantitation
of Recombinant Protein in Whole Cells and Cell Extracts via Solid-State NMR
Spectroscopy”, Biochemistry, 52, 4285-4287 (2013) – highlighted on Biochemistry website.
21.
U. Ghosh, L. Xie, and D. P. Weliky, “Detection
of Closed Influenza Virus Hemagglutinin Fusion Peptide Structures in Membranes
by Backbone 13CO-15N Rotational-Echo Double-Resonance
Solid-State NMR”, Journal of Biomolecular
NMR, 55, 139-146 (2013).
22.
L. Xie, U. Ghosh, S. D. Schmick, and
D. P. Weliky, “Residue-Specific Membrane Location of Peptides and Proteins
Using Specifically and Extensively Deuterated Lipids and 13C-2H
Rotational-Echo Double-Resonance Solid-State NMR”, Journal of Biomolecular NMR, 55,
11-17 (2013).
23.
E. P. Vogel, J. Curtis-Fisk, K. M. Young, and D.
P. Weliky, “Solid-State Nuclear Magnetic Resonance (NMR) Spectroscopy of Human
Immunodeficiency Virus gp41 Protein that includes the Fusion Peptide: NMR
Detection of Recombinant Fgp41 in Inclusion Bodies in Whole Bacterial Cells and
NMR Structural Characterization of Purified and Membrane-Associated Fgp41”, Biochemistry, 50, 10013-10026 (2011).
24.
K. Sackett, A. TerBush,
and D. P. Weliky, “HIV Six-Helix Bundle Constructs Induce Rapid Vesicle Fusion
at pH 3.5 and Little Fusion at pH 7.4: Understanding pH Dependence of Protein
Aggregation, Membrane Binding, and Electrostatics, and Implications for
HIV-Host Cell Fusion”, European
Biophysics Journal, 40, 489-502
(2011).
25.
S. D. Schmick and D. P. Weliky, “Major Antiparallel and Minor Parallel b Sheet Populations
Detected in the Membrane-Associated Human Immunodeficiency Virus Fusion Peptide”,
Biochemistry, 49, 10623-10635 (2010).
26.
S. Tristram-Nagle, R. Chan, E. Kooijman,
P. Uppamoochikkal, W. Qiang, D. P. Weliky, and J. F.
Nagle, “HIV Fusion Peptide Penetrates,
Disorders, and Softens T-cell Membrane Mimics”, Journal of Molecular Biology, 402,
139-153 (2010).
27.
I. Chung, D. Holmes, D. P. Weliky, and M. G.
Kanatzidis, “[P3Se7]3–: A Phosphorus-Rich
Square-Ring Selenophosphate”, Inorganic Chemistry, 49,
3092-3094 (2010).
28.
K. Sackett, M. J. Nethercott, R. F.
Epand, R. M. Epand, D. R. Kindra, Y. Shai, and D. P. Weliky, “Comparative
Analysis of Membrane-Associated Fusion Peptide Secondary Structure and Lipid
Mixing Function of HIV gp41 Constructs that Model the Early Pre-Hairpin
Intermediate and Final Hairpin Conformations”, Journal of Molecular Biology, 397,
301-315 (2010).
29.
C. M. Gabrys, R. Yang, C. M. Wasniewski, J. Yang, C.
G. Canlas, W. Qiang, Y. Sun, and D. P. Weliky, “Nuclear Magnetic Resonance
Evidence for Retention of Lamellar Membrane Phase with Curvature in the
Presence of Large Quantities of the HIV Fusion Peptide”, Biochimica et Biophysica Acta, 1798, 194-201 (2010).
30.
N. Huarte, J. L. Nieva, S. Nir and
D. P. Weliky, “Induced Perturbations and Adopted Conformations in Membranes by
the HIV-1 Fusion Peptide”, In Membrane-Active
Peptides: Methods and Results on Structure and Function, M. A. R. B. Castanho, Editor, International University Line:La Jolla, 2009, pp. 565-596.
31.
I. Chung, J.-H. Song, M. G. Kim, C. D. Malliakas, A.
L. Karst, A. J. Freeman, D. P. Weliky, and M. G. Kanatzidis,
“The Tellurophosphate
K4P8Te4: Phase-Change Properties, Exfoliation,
Photoluminescence in Solution and Nanospheres”, Journal of the American Chemical Society, 131, 16303-16312 (2009).
32.
Y. Sun and D. P. Weliky, “13C-13C
Correlation Spectroscopy of Membrane-Associated Influenza Virus Fusion Peptide
Strongly Supports a Helix-Turn-Helix Motif and Two Turn Conformations”, Journal of the American Chemical Society,
131, 13228-13229 (2009).
33.
W. Qiang, Y. Sun, and D. P.
Weliky, “A Strong Correlation Between Fusogenicity
and Membrane Insertion Depth of the HIV Fusion Peptide”, Proceedings of the National Academy of Sciences of the U.S.A., 106, 15314-15319 (2009).
34.
K. Sackett, M. J. Nethercott,
Y. Shai, and D. P. Weliky, “Hairpin Folding of HIV gp41 Abrogates Lipid Mixing
Function at Physiologic pH and Inhibits Lipid Mixing by Exposed gp41
Constructs”, Biochemistry, 48, 2714-2722 (2009).
35.
W. Qiang and D. P. Weliky,
“HIV Fusion Peptide and its
Cross-Linked Oligomers: Efficient Syntheses, Significance of the Trimer in
Fusion Activity, Correlation of b Strand Conformation with Membrane
Cholesterol, and Proximity to Lipid Headgroups”, Biochemistry, 48,
289-301 (2009).
36.
J. Curtis-Fisk, R. M.
Spencer, and D. P. Weliky, “Native Conformation at Specific Residues in
Recombinant Inclusion Body Protein in Whole Cells Determined with Solid-State
Nuclear Magnetic Resonance Spectroscopy”, Journal
of the American Chemical Society, 130,
12568-12569 (2008). – Featured in Chemical and Engineering News, 86, 31 (2008).
37.
J. Curtis-Fisk, R. M.
Spencer, and D. P. Weliky, “Isotopically
Labeled Expression in E. coli, Purification, and Refolding of the Full Ectodomain of the Influenza
Virus Membrane Fusion Protein”, Protein
Expression and Purification, 61,
212-219 (2008).
38.
W. Qiang, M. L Bodner, and D.
P. Weliky, “Solid-State NMR
Spectroscopy of HIV Fusion Peptides Associated with Host-Cell-Like Membranes:
2D Correlation Spectra and Distance Measurements Support a Fully Extended
Conformation and Models for Specific Antiparallel Strand Registries”, Journal of the American Chemical Society,
130, 5459-5471 (2008).
39.
M. A. Gave, K. M. Johnson, M.
G. Kanatzidis, and D. P. Weliky, “Improved Resolution and Detection of 31P-Tl
J-Couplings at 21 T in 31P Magic Angle Spinning Spectra of Inorganic
Compounds Containing Tl/Bi/P/S”, Solid
State Nuclear Magnetic Resonance, 33,
12-15 (2008).
40.
M. L. Bodner, C. M. Gabrys,
J. O. Struppe, and D. P. Weliky, “13C-13C and 15N-13C
Correlation Spectroscopy of Membrane-Associated and Uniformly Labeled HIV and
Influenza Fusion Peptides: Amino Acid-Type Assignments and Evidence for
Multiple Conformations”, Journal of
Chemical Physics, 128, 052319
(2008).
41.
Z. Zheng, W. Qiang, and D. P.
Weliky, “Investigation of Finite-Pulse Radiofrequency-Driven Recoupling Methods
for Measurement of Intercarbonyl Distances in Polycrystalline
and Membrane-Associated HIV Fusion Peptide Samples”, Magnetic Resonance in Chemistry, 45, S247-S260 (2007).
42.
C. M. Gabrys and D. P.
Weliky, “Chemical Shift Assignment and Structural Plasticity of a HIV Fusion
Peptide Derivative in Dodecylphosphocholine
Micelles”, Biochimica et Biophysica
Acta-Biomembranes, 1768,
3225-3234 (2007).
43.
M. A. Gave, D. P. Weliky,
and M. G. Kanatzidis, “New Potassium Bismuth Thiophosphates Including the
Modulated K1.5Bi2.5(PS4)3”, Inorganic Chemistry, 46, 11063 -11074 (2007).
44.
I. Chung, J. I. Jang, M. A. Gave, D.
P. Weliky, and M. G. Kanatzidis, “Low Valent
Phosphorus in the Molecular Anions [P5Se12]5–
and b-[P6Se12]4–:
Phase Change Behavior and Near Infrared Second Harmonic Generation”, Chemical Communications, 4998-5000
(2007).
45.
I. Chung, C. D. Malliakas, J. I. Jang,
C. G. Canlas, D. P. Weliky, and M. G. Kanatzidis, “Helical Polymer 1/¥[P2Se62–]:
Strong Second Harmonic Generation Response and Phase-Change Properties of its K
and Rb Salts”, Journal of the American
Chemical Society, 129,
14996-15006 (2007).
46.
M. A. Gave, C. G. Canlas, I.
Chung, R. G. Iyer, M. G. Kanatzidis, and D. P. Weliky, “Cs4P2Se10:
A New Compound Discovered with the Application of Solid State and High
Temperature NMR”, Journal of Solid State
Chemistry, 180, 2877-2884
(2007).
47.
J. Curtis-Fisk, C. Preston,
Z. Zheng, R. M. Worden, and D. P. Weliky, “Solid-State NMR Structural
Measurements on the Membrane-Associated Influenza Fusion Protein Ectodomain”, Journal of the American Chemical Society,
129, 11320-11321 (2007).
48.
W. Qiang, J. Yang, and D. P. Weliky, “Solid-State
Nuclear Magnetic Resonance Measurements of HIV Fusion Peptide to Lipid
Distances Reveal the Intimate Contact of b Strand Peptide with Membranes and the
Proximity of the Ala-14-Gly-16 Region with Lipid Headgroups”, Biochemistry, 46, 4997-5008 (2007).
49.
M. A. Gave, C. D. Malliakas, D. P. Weliky,
and M. G. Kanatzidis, “Wide Compositional and Structural Diversity in the
System Tl/Bi/P/Q (Q = S, Se) and Observation of Vicinal P-Tl J Coupling
in the Solid State”, Inorganic Chemistry,
46, 3632-3644 (2007).
50.
Z. Zheng, R. Yang, M. L. Bodner, and D. P.
Weliky, “Conformational Flexibility and Strand Arrangements of the
Membrane-Associated HIV Fusion Peptide Trimer Probed by Solid-State NMR Spectroscopy”,
Biochemistry, 45,
12960-12975 (2006).
51.
I. Chung, A. L. Karst, D. P. Weliky, and M.
G. Kanatzidis, “[P6Se12]4–: A Phosphorus-Rich Selenophosphate with Low-Valent P Centers”, Inorganic Chemistry, 45, 2785-2787 (2006).
52.
O. Palchik, R. G.
Iyer, C. G. Canlas, D. P. Weliky, and M. G. Kanatizidis,
“K10M4M4¢S17 (M = Mn,
Fe, Co, Zn; M¢
= Sn, Ge) and Cs10Cd4Sn4S17:
Compounds with a Discrete Supertetrahedral Cluster”, Z.
Anorg. Allg. Chem., 630,
2237-2247 (2004).
53.
R. Yang, M. Prorok, F. J. Castellino, and D. P. Weliky, “A Trimeric HIV-1 Fusion
Peptide Construct Which Does Not Self-Associate in Aqueous Solution and Which
Has Fifteen-Fold Higher Membrane Fusion Rate”, Journal of the American
Chemical Society, 126, 14722-14723 (2004).
54.
C. M. Wasniewski, P. D. Parkanzky, M. L. Bodner, and D.
P. Weliky, “Solid-State Nuclear Magnetic Resonance Studies of HIV and Influenza
Fusion Peptide Orientations in Membrane Bilayers Using Stacked Glass Plate
Samples”, Chemistry and Physics of Lipids, 132, 89-100 (2004).
55.
J. Yang, M. Prorok, F. J. Castellino,
and D. P. Weliky, “Oligomeric Structure of the Membrane-Bound HIV-1 Fusion
Peptide Formed From Soluble Monomers”, Biophysical Journal, 87,
1951-1963 (2004).
56.
I. Chung, J. Do, C. G. Canlas, D. P. Weliky, and M. G. Kanatizidis, “APSe6 (A = K, Rb, and Cs):
Polymeric Selenophosphates with Reversible
Phase-Change Properties”, Inorganic Chemistry, 43, 2762-2764 (2004).
57.
M. L. Bodner, C. M. Gabrys, P. D. Parkanzky, J. Yang, C.
A. Duskin, and D. P. Weliky, “Temperature Dependence
and Resonance Assignment of 13C NMR Spectra of Selectively and
Uniformly Labeled Fusion Peptides Associated with Membranes”, Magnetic
Resonance in Chemistry, 42, 187-194 (2004).
58.
J. Yang and D. P. Weliky, “Solid State Nuclear Magnetic
Resonance Evidence for Parallel and Antiparallel Strand Arrangements in the
Membrane-Associated HIV-1 Fusion Peptide”, Biochemistry, 42,
11879-11890 (2003).
59.
R. J. DiCosty, D. P.
Weliky, S. J. Anderson, and E. A. Paul, “15N-CPMAS Nuclear Magnetic
Resonance Spectroscopy and Biological Stability of Soil Organic Nitrogen in
Whole Soil and Particle-Size Fractions”, Organic Geochemistry, 34,
1635-1650 (2003).
60.
C. G. Canlas, R. B. Muthukumaran,
M. G. Kanatzidis, and D. P. Weliky, “Investigation
of Longitudinal 31P Relaxation in Metal Selenophosphate
Compounds”, Solid State Nuclear Magnetic Resonance, 24, 110-122
(2003).
61.
C. G. Canlas, M. G. Kanatzidis, and D. P.
Weliky, “31P Solid State NMR Studies of Metal Selenophosphates
Containing [P2S6]4-, [P4S10]4-,
[PSe4]3-, [P2Se7]4-,
and [P2Se9]4- Ligands”, Inorganic
Chemistry, 42, 3399-3405 (2003).
62.
R. Yang, J. Yang, and D. P. Weliky, “Synthesis,
Enhanced Fusogenicity, and Solid State NMR Measurements
of Cross-Linked HIV-1 Fusion Peptides”, Biochemistry, 42,
3527-3535 (2003).
63.
C. M. Gabrys, J. Yang, and D. P. Weliky, “Analysis of
Local Conformation of Membrane-Bound and Polycrystalline Peptides by
Two-Dimensional Slow-Spinning Rotor-Synchronized MAS Exchange Spectroscopy”, Journal
of Biomolecular NMR, 26, 49-68 (2003).
64.
J. Yang, P. D. Parkanzky, M. L. Bodner, C. G. Duskin, and D. P. Weliky, “Application of REDOR Subtraction
for Filtered MAS Observation of Labeled Backbone Carbons of Membrane-Bound
Fusion Peptides”, Journal of Magnetic Resonance, 159, 101–110
(2002).
65.
K. K. Rangan, P. N. Trikalitis,
C. Canlas, T. Bakas, D. P. Weliky, and M. G. Kanatzidis,
“Hexagonal Pore Organization in Mesostructured Metal Tin Sulfides Built with
[Sn2S6]4- Clusters”, Nano Letters, 2,
513 – 517 (2002).
66.
J. A. Aitken, C. Canlas, D. P. Weliky, and
M. G. Kanatzidis, “[P2S10]4-: A Novel Polythiophosphate Anion Containing a Tetrasulfide
Fragment”, Inorganic Chemistry, 40, 6496-6498 (2001).
67.
J. Yang, C. M. Gabrys, and D. P. Weliky, “Solid
State Nuclear Magnetic Resonance Evidence for an Extended b Strand Conformation of the Membrane-Bound
HIV-1 Fusion Peptide”, Biochemistry, 40, 8126-8137 (2001).
68.
J. Yang, P. D. Parkanzky, B. A. Khunte, C. G. Canlas, R. Yang,
C. M. Gabrys, and D. P. Weliky, "Solid State NMR Measurements of
Conformation and Conformational Distributions in the Membrane-Bound HIV-1
Fusion Peptide", Journal of
Molecular Graphics and Modelling, 19, 129-135 (2001).
69.
J. J. Balbach, J. Yang, D.
P. Weliky, P. J. Steinbach, V. Tugarinov, J. Anglister, and R. Tycko, "Probing Hydrogen Bonds in
the Antibody-Bound HIV-1 gp120 V3 Loop by Solid State NMR REDOR
Measurements", Journal of Biomolecular NMR, 16, 313-327
(2000).
If you have comments or suggestions,
email us at weliky@chemistry.msu.edu
This
page created on June 5, 1998 and last modified on May 14, 2019!
![]()

![]()