X-Ray Fluorescence Introduction |
 |
What is X-ray Fluorescence?
X-ray fluorescence (XRF) is an analytical technique that can be used to determine the chemical composition of a wide variety of sample types including solids, liquids, slurries and loose powders. X-ray fluorescence is also used to determine the thickness and composition of layers and coatings. It can analyze elements from beryllium (Be) to uranium (U) in concentration ranges from 100 wt% to sub-ppm levels. Some of the benefits of XRF analysis
- XRF analysis is a robust technique
- Combines high precision and accuracy with straightforward sampling
- Fast sample preparation
- Provides both qualitative and quantitative information on a sample, easy combination of this ‘what?’ and ‘how much?’ information also makes rapid screening (semi-quantitative) analysis possible
- Non-destructive
- Liquid, and solids can be measured
Simple Principles Behind XRF
XRF is an atomic emission method, similar in this respect to optical emission spectroscopy (OES), ICP and neutron activation analysis (gamma spectroscopy). Such methods measure the wavelength and intensity of ‘light’ (X-rays in this case) emitted by energized atoms in the sample. In XRF, irradiation by a primary X-ray beam from an X-ray tube, causes emission of fluorescent X-rays with discrete energies characteristic of the elements present in the sample.
In the XRF spectrometer, samples are irradiated with x-rays that provide sufficient energy to remove core electrons from atoms present in the sample. An atom missing a core electron is unstable and will reach a more stable electronic configuration by filling the hole with an electron from a shell having a higher principal quantum number n. Energy must be conserved when the atom reaches a lower energy electronic configuration by filling the hole left by ionization. Consequently, the excess energy is released as emission, which falls in the x-ray region of the electromagnetic spectrum and is known as x-ray fluorescence. The energy of the emitted photon corresponds to ∆E = Ef – Ei , where Ei is the energy of the orbital in which the electron undergoing the transition initially resided and Ef is the energy of the electron after it fills the hole. Transitions in an XRF spectrum are identified using a capital letter followed by a lower case Greek letter (ex. Kα, Kβ, Lβ,...) to identify the core shell that initially contained the hole and the change in the principal quantum number of the electron that filled the hole. In this notation, the letters K, L, M, N,... represent a hole in the shell having the principal quantum numbers n = 1, 2, 3, 4... Alternately, the letters K, L, M, N... correspond to the principal quantum number of the electron following emission of x-ray fluorescence. The lower case Greek letter indicates the change in principal quantum number ∆n = nf – ni associated with the XRF transition, where the letters α,β,γ, δ,... correspond to ∆n = -1, -2, -3, -4….
Sometimes, as the atom returns to its stable condition, instead of emitting a characteristic x-ray it transfers the excitation energy directly to one of the outer electrons, causing it to be ejected from the atom. The ejected electron is called an “Auger” electron. This process is a competing process to XRF. Auger electrons are more probable in the low Z elements than in the high Z elements.
X-Ray Fluorescence Process Example: Titanium Atom (Ti=22)
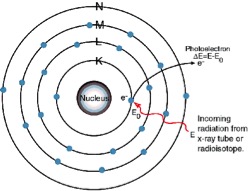
1. An electron in the K shell is ejected from the atom by an external primary excitation x-ray, creating a vacancy.
The K Lines
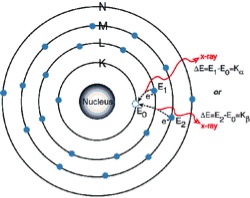
2. An electron from the L or M shell “jumps in” to fill the vacancy. In the process, it emits a characteristic x-ray unique to this element and in turn, produces a vacancy in the L or M shell.
The L Lines
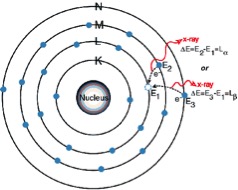
3. When a vacancy is created in the L shell by either the primary excitation x-ray or by the previous event, an electron from the M or N shell “jumps in” to occupy the vacancy. In this process, it emits a characteristic x-ray unique to this element and in turn, produces a vacancy in the M or N shell.
“Auger” Electron
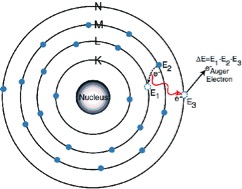
The excitation energy from the inner atom is transferred to one of the outer electrons causing it to be ejected from the atom.
Please note: Use of this instrument for Chemnistry and Forensic Chemistry may be authorized by Dr. Kathy Severn, who is then responsible for training and safety.