Repurposing Enzymes to Construct Biosynthetic Pathways toward Non-natural Bioactive Compounds
Biocatalysis of β-Amino Acids by Aminomutases
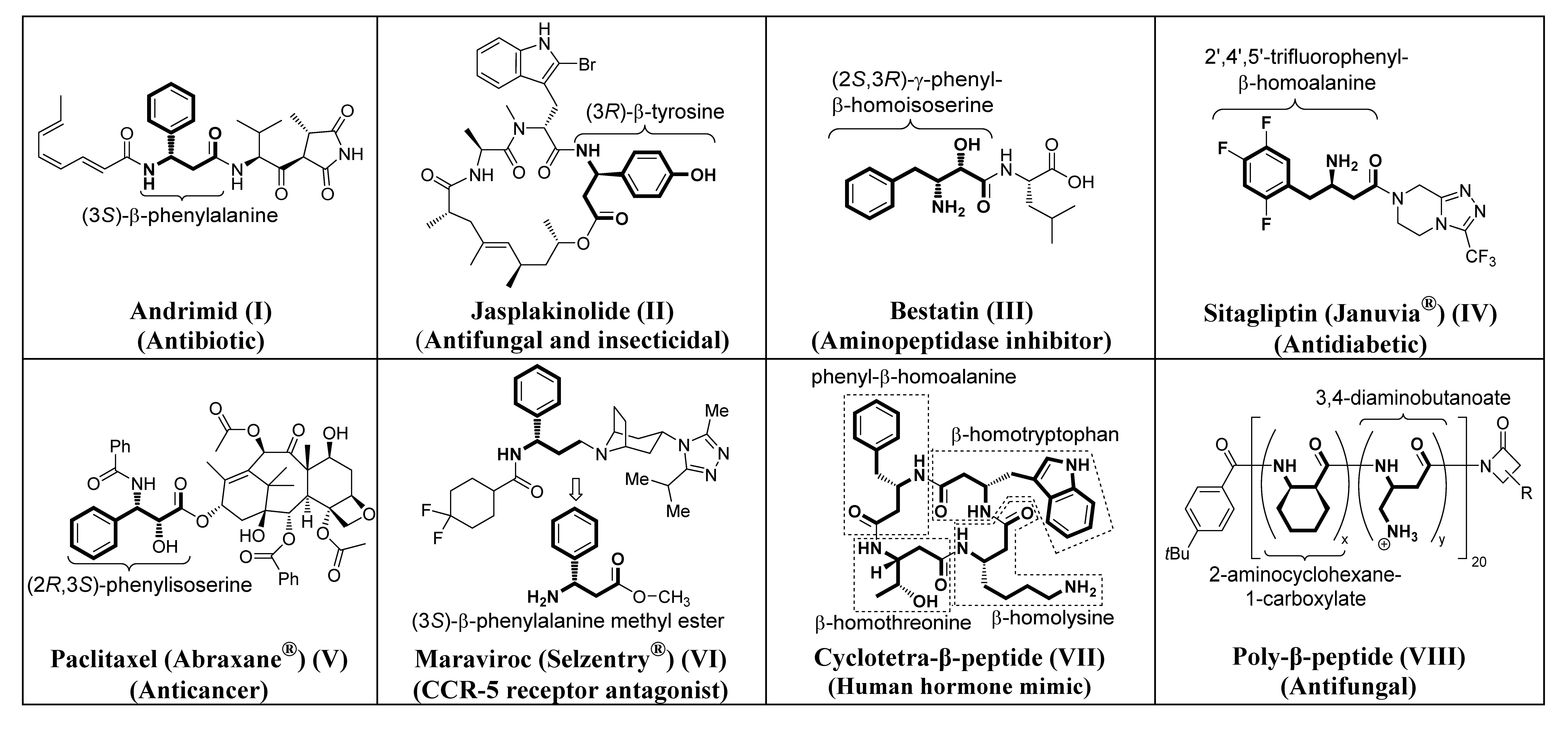
Importance of β-Amino Acids as Bioactive Products
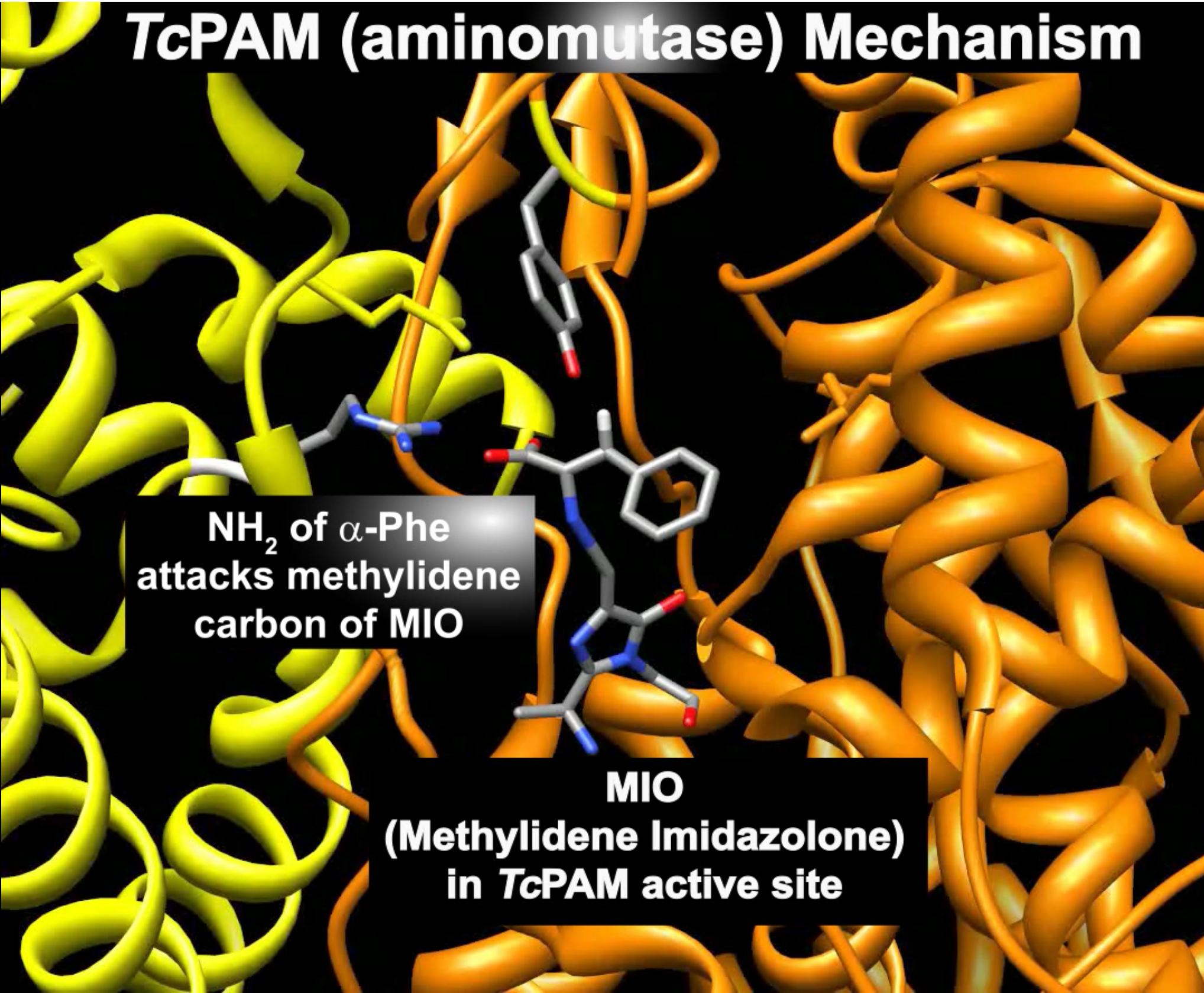
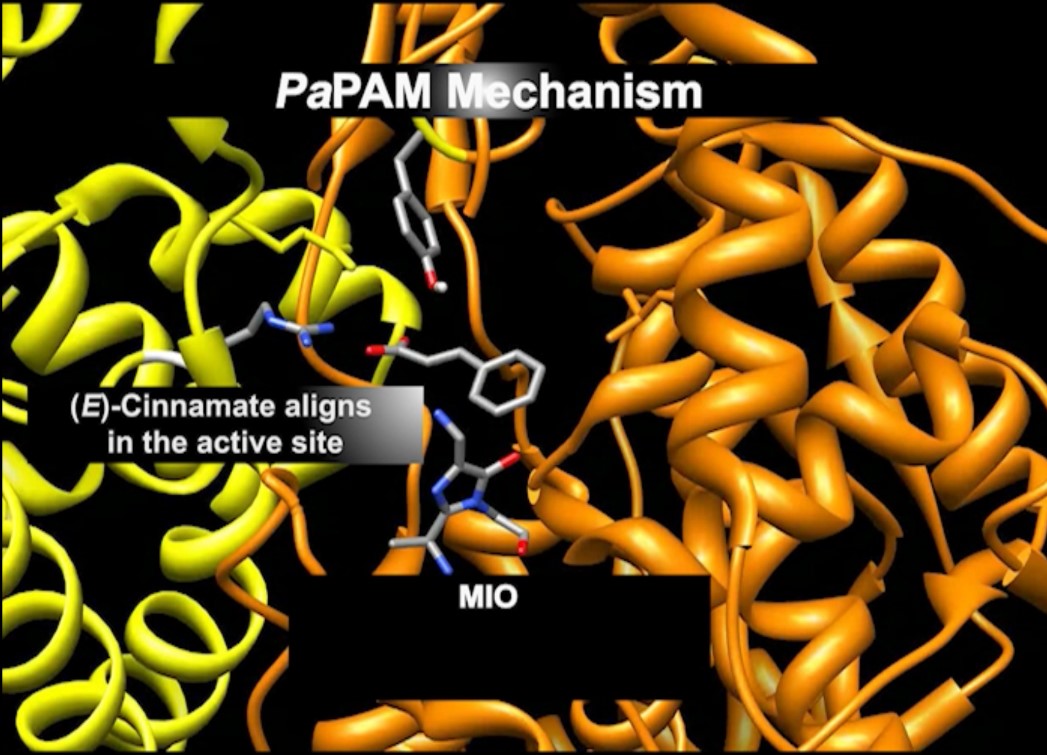
Mechanisms of Aminomutases in the Biocatalysis of β-Amino Acids
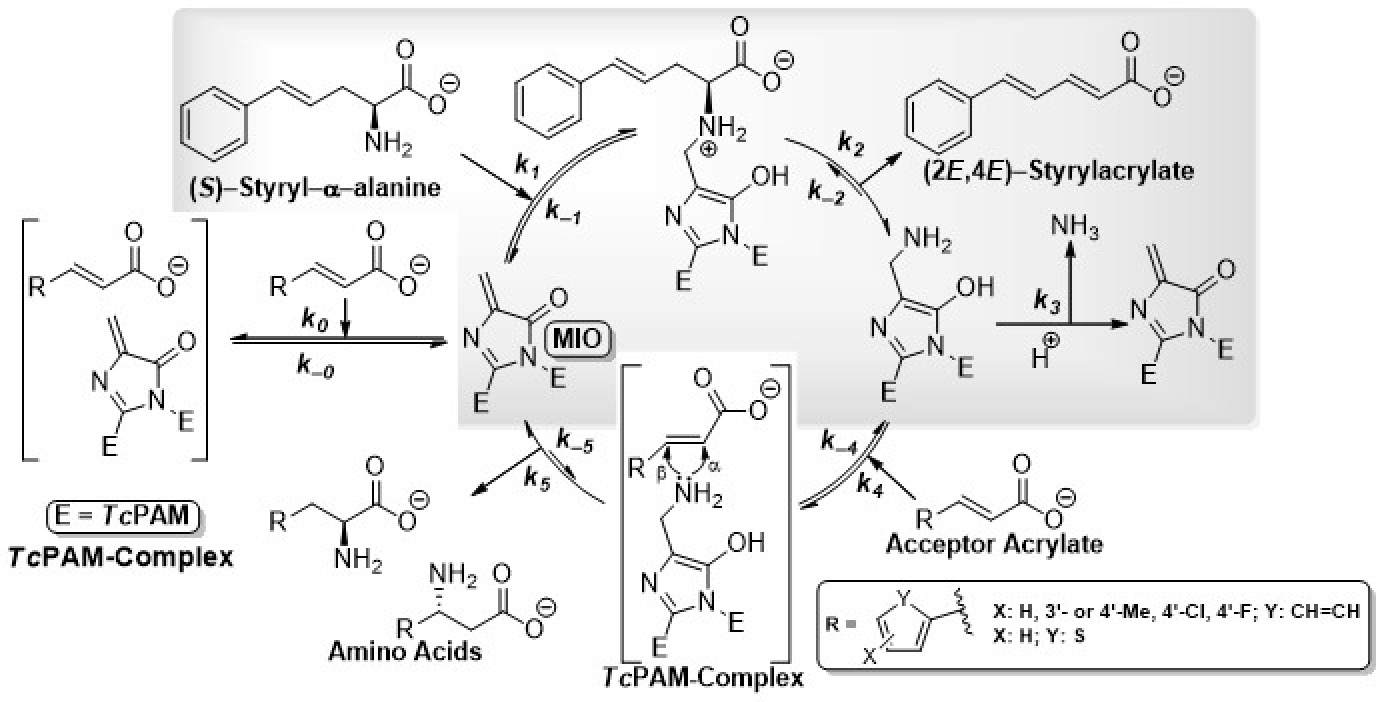
A Taxus phenylalanine aminomutase (TcPAM) on the biosynthetic pathway of the antineoplastic drug paclitaxel converts (2S)-α-Phe to (3R)-β-Phe in Taxus plants. To understand how to use TcPAM chemistry to make β-amino acids, it is necessary to understand the subtleties of its mechanism. TcPAM rotates/flips its cinnamate intermediate 180° before product release. By contrast, PaPAM from Pantoea agglomerans, that makes andrimid, must hold the intermediate stationary (see videos below).
Burst-Phase Analysis. The TcPAM forms a transient MIO-NH2 adduct with a finite lifetime. The lifetime of adduct was unknown for TcPAM or any of the several enzymes in this family until we used stopped-flow monitoring of product release to measure the exponential burst phase at pre-steady state (below).
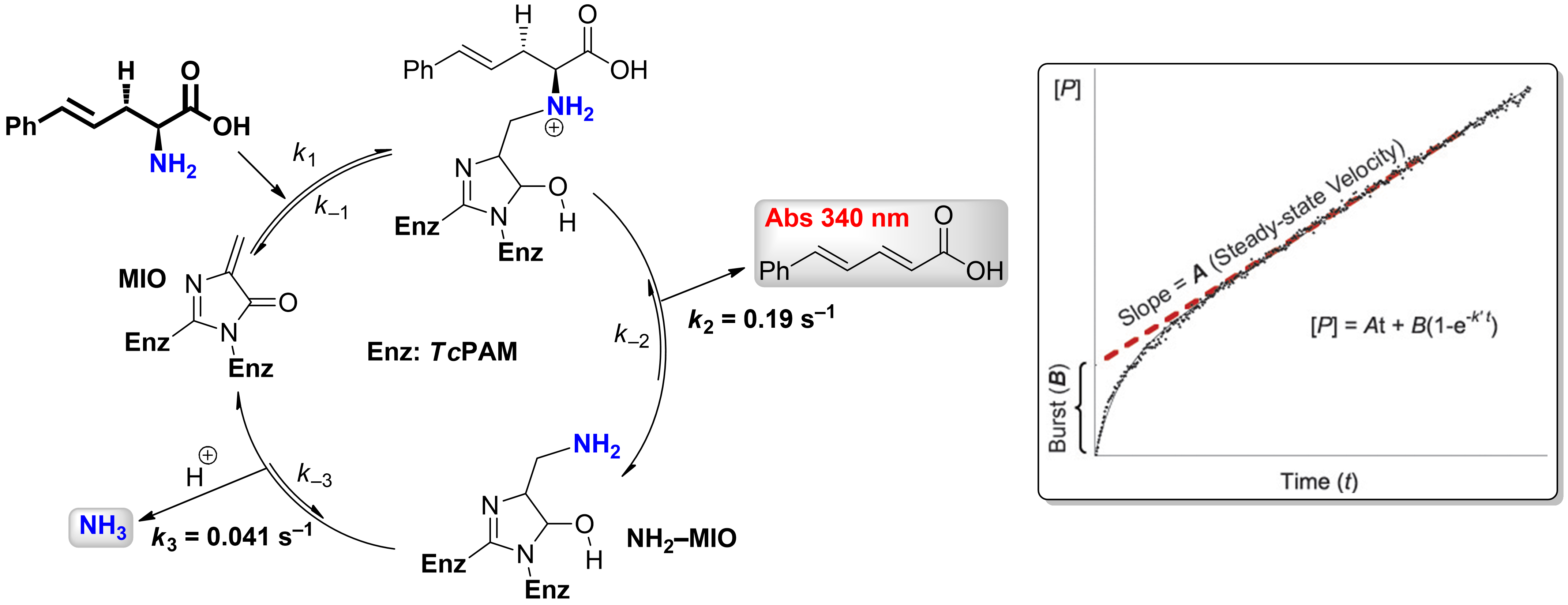
Catalytic Cycle for Amino Acid Biocatalysis via Transamination. Kinetic Model for Transaminase Reaction Catalyzed by TcPAM.
Hammett correlations. Electron-withdrawing and -donating substituents on the aryl ring changed the rate-determining step of an aminomutase (PaPAM)-catalyzed reaction. Studies on this family of enzymes (class-I lyase) that includes PaPAM began in 1967. Our group, for the first time, showed that the rate-determining step of a class-1 lyase aminomutase is sensitive to the electronics of the substituents.
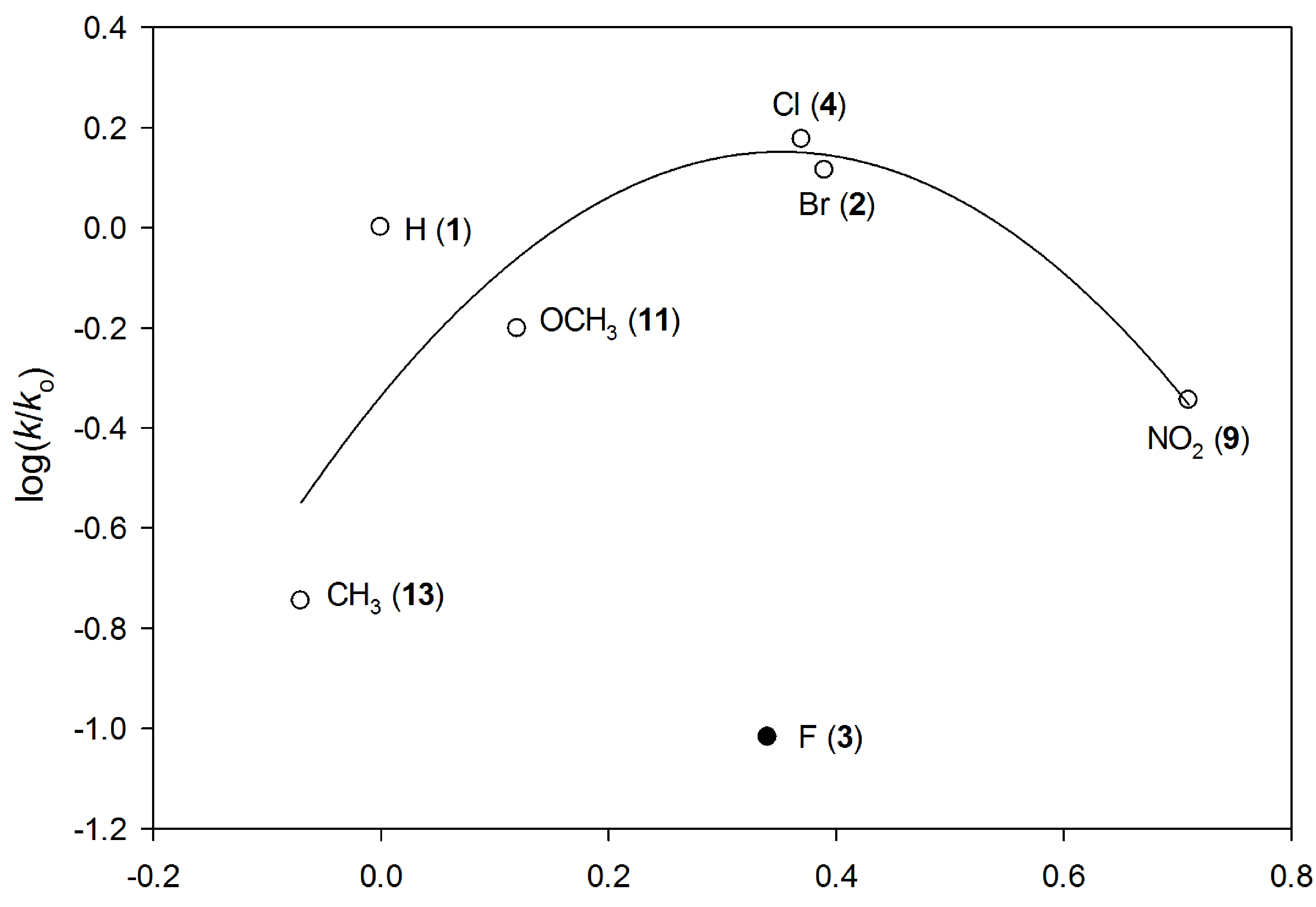
The influence of the substituents on the kcat of PaPAM revealed a concave-down or a downward break in correlations with Hammett substituent constants (σ). The trend suggests the rate-determining step changes from the elimination to the amination step based on the electronic properties of the substituent.
 |
|---|