Biocatalysis of Arylserines and Arylisoserines from Epoxides
Over several decades, many accounts on methylidene imidazolone (MIO)-dependent biocatalysts described how these enzymes add ammonia (at high concentrations from ammonium salts) across arylacrylate double bonds to make α- and, more recently, β-arylalanines. β-Amino acids are excellent building blocks of valuable bioactive compounds. These biocatalysis efforts have even drawn contributions from Nobel Laureate (Ben Feringa/ Wiki) to help dissect the dynamics of the catalysis among this family of enzymes.
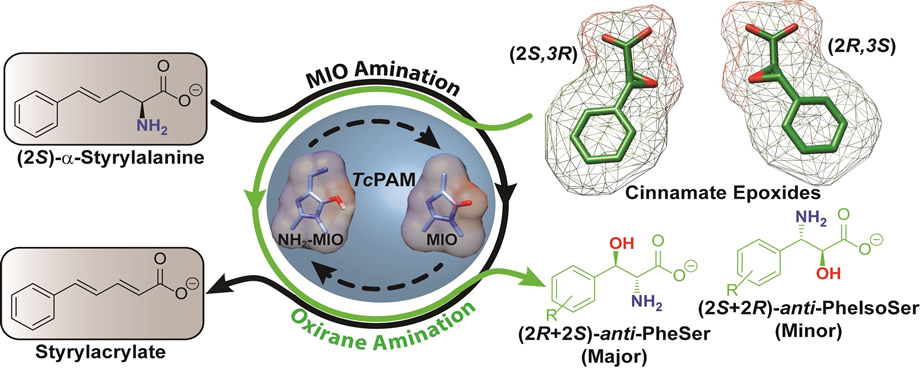
We diverge from this fixed program of aminating cinnamic acids and describe a first account of employing an MIO-aminomutase (TcPAM) from Taxus plants to convert previously untested epoxy acid substrates to bifunctional hydroxy amino acids. Arylserines were made more abundantly over the arylisoserines when styrylalanine was used instead of ammonium salts, to selectively transaminate the MIO group of TcPAM. This mild amine group delivery to the oxirane avoided nonenzymatic ring-opening of the epoxides.
Exploring the scope of an α/β-aminomutase for the amination of cinnamate epoxides to arylserines and arylisoserines
Shee, P.K.; Ratnayake, N.D.; Walter, T.; Goethe, O.; Onyeozili, E.N.; Walker, K. D. ACS Catal. 2019, 9, 7418-7430.
Shee, P.K.; Ratnayake, N.D.; Walter, T.; Goethe, O.; Onyeozili, E.N.; Walker, K. D. ACS Catal. 2019, 9, 7418-7430.

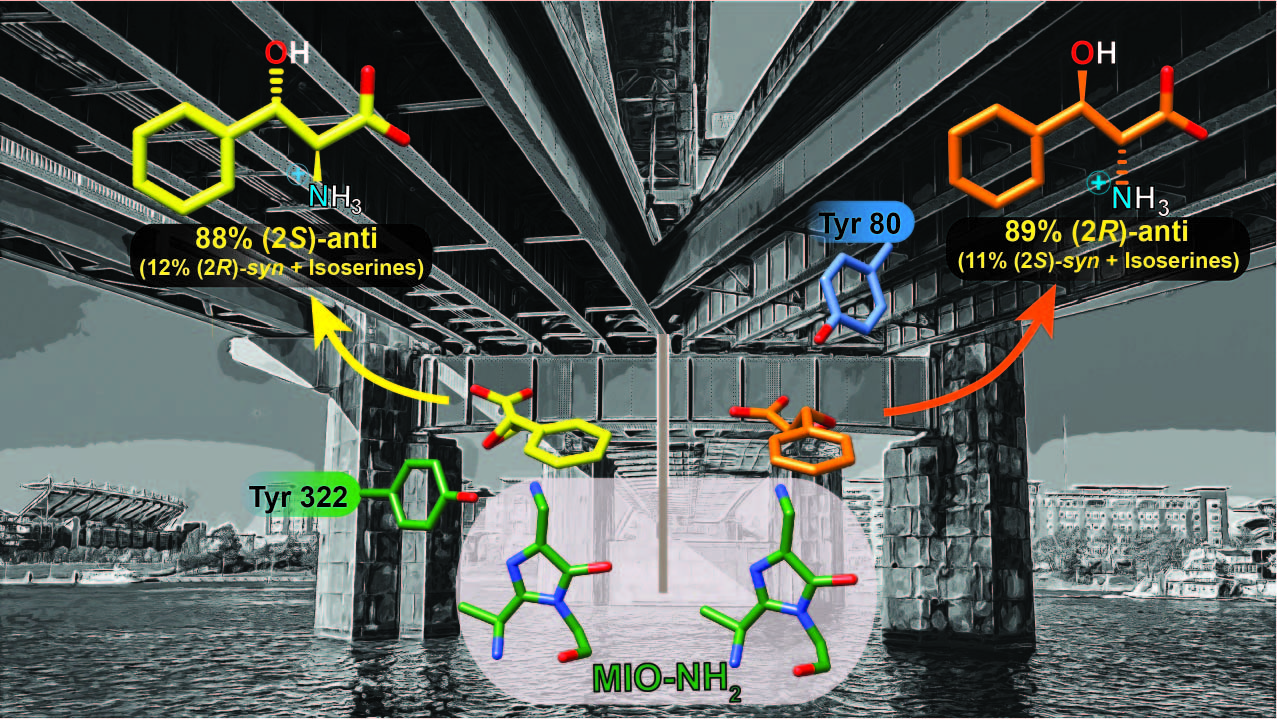
Intermolecular amine transfer to enantioenriched trans-3-phenylglycidates by an α/β-aminomutase to access both anti-phenylserine isomers
Shee, P.K.; Yan, H.; Walker, K. D. ACS Catal. 2020, 10, 15071-15082.
Shee, P.K.; Yan, H.; Walker, K. D. ACS Catal. 2020, 10, 15071-15082.

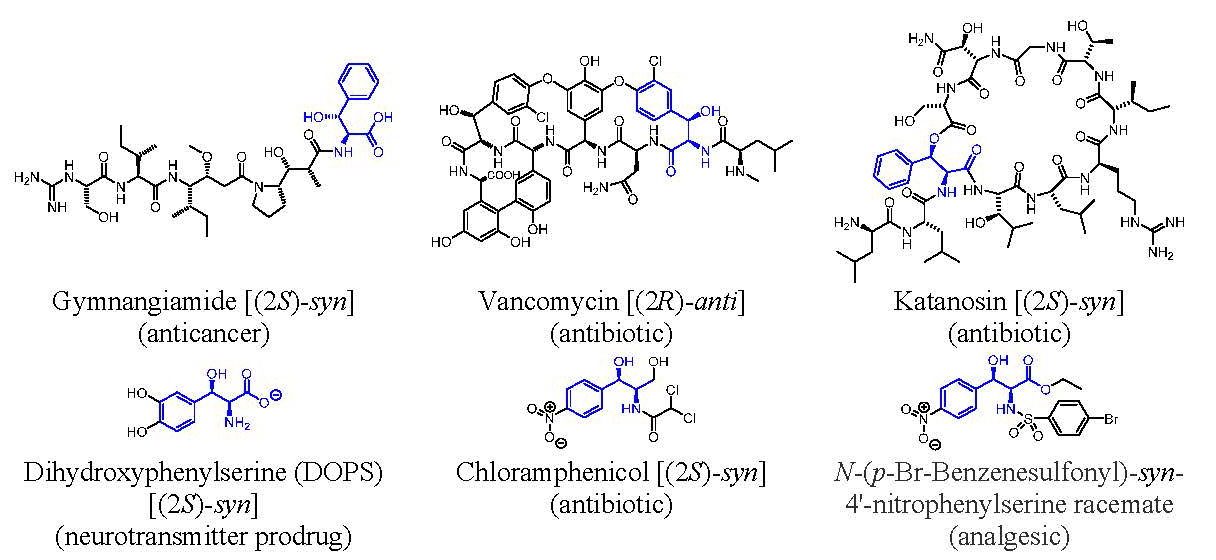
Importance of Serines and Isoserines
|