Metal Boron Chemistry
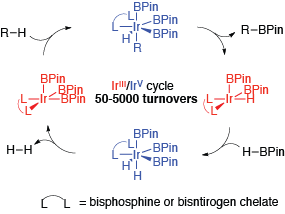 We have a long-standing interest in the organometallic chemistry of simple boron ligands bond to metals. The bonding in these complexes is fundamentally interesting and the metal boron bonds in these complexes have a unique reaction chemistry that enables unique functionalizations of organic molecules under mild conditions. For example, our group pioneered the Ir-catalyzed cross-coupling of C-H and B-H bonds to form new B-C bonds with the liberation of H2as the only byproduct. This marked a new synthesis of organoboron compounds from hydrocarbons and boranes, bypassing hydrocarbon halogenation steps that were previously required. Our collaboration with the Maleczka group has expanded the scope of this reaction to the point that it is a viable synthetic method. In 2008, these accomplishments were recognized with the award of the Presidential Green Chemistry Challenge Award to the Maleczka/Smith team.
We have a long-standing interest in the organometallic chemistry of simple boron ligands bond to metals. The bonding in these complexes is fundamentally interesting and the metal boron bonds in these complexes have a unique reaction chemistry that enables unique functionalizations of organic molecules under mild conditions. For example, our group pioneered the Ir-catalyzed cross-coupling of C-H and B-H bonds to form new B-C bonds with the liberation of H2as the only byproduct. This marked a new synthesis of organoboron compounds from hydrocarbons and boranes, bypassing hydrocarbon halogenation steps that were previously required. Our collaboration with the Maleczka group has expanded the scope of this reaction to the point that it is a viable synthetic method. In 2008, these accomplishments were recognized with the award of the Presidential Green Chemistry Challenge Award to the Maleczka/Smith team.
Polymer Chemistry
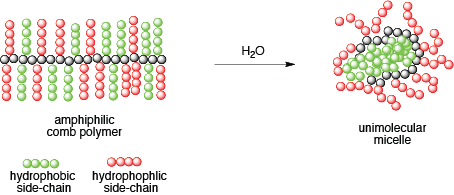 Polylactic acids (PLA) are a family of polymers that have attracted interest for applications that range from renewable plastics to drug delivery. We have shown that modifications to the polymer side-chains can create degradable materials that mimic polystyrene and natural rubber. More recently we have designed degradable materials that can participate in "click" reactions. This enables the synthesis of new materials with widely ranging properties. For example, we can create amphiphilic polymer brushes that form unimolecular micelles by attaching hydrophilic and hydrophobic arms to the polymer backbone. We are currently evaluation these materials for applications that range from drug delivery to protein stabilization.
Polylactic acids (PLA) are a family of polymers that have attracted interest for applications that range from renewable plastics to drug delivery. We have shown that modifications to the polymer side-chains can create degradable materials that mimic polystyrene and natural rubber. More recently we have designed degradable materials that can participate in "click" reactions. This enables the synthesis of new materials with widely ranging properties. For example, we can create amphiphilic polymer brushes that form unimolecular micelles by attaching hydrophilic and hydrophobic arms to the polymer backbone. We are currently evaluation these materials for applications that range from drug delivery to protein stabilization.
Energy Storage and Conversion
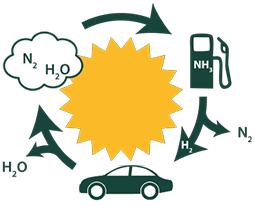 As global population and energy consumption continue to increase, it is critical that humankind transitions to renewable resources like solar and wind energy. Because these sources are intermittent, it is essential that the energy be stored so it can be accessed on demand. Expect for nuclear fuels, nothing rivals the energy density that can be stored in chemical bonds. Recent advances in photochemical water splitting are making the prospects for renewable hydrogen bright. Because hydrogen is a low-density gas, it is desirable to store it in a more energy dense material. We have targeted liquid ammonia as a hydrogen carrier because (i) it has a high energy density, (ii) it is produced on large scale from hydrogen and nitrogen, the most abundant gas in the atmosphere, and (ii) when oxidized it produces nitrogen and water, leaving no carbon footprint. We are exploring new avenues for synthesizing ammonia and extracting the energy stored in its chemical bonds.
As global population and energy consumption continue to increase, it is critical that humankind transitions to renewable resources like solar and wind energy. Because these sources are intermittent, it is essential that the energy be stored so it can be accessed on demand. Expect for nuclear fuels, nothing rivals the energy density that can be stored in chemical bonds. Recent advances in photochemical water splitting are making the prospects for renewable hydrogen bright. Because hydrogen is a low-density gas, it is desirable to store it in a more energy dense material. We have targeted liquid ammonia as a hydrogen carrier because (i) it has a high energy density, (ii) it is produced on large scale from hydrogen and nitrogen, the most abundant gas in the atmosphere, and (ii) when oxidized it produces nitrogen and water, leaving no carbon footprint. We are exploring new avenues for synthesizing ammonia and extracting the energy stored in its chemical bonds.
 Mission
Mission

