Total Synthesis of Natural Products
Marine sponge metabolites represent a highly diverse and structurally complex natural library of compounds with remarkable biological and medicinal properties. Students in the group will develop novel heterocyclic methodologies that enable the efficient synthesis of natural products. Our research aims to conquer both the natural products and abridges scaffolds by total synthesis, unravel their biological properties and optimize the abridged scaffolds as medicinal leads or biochemical tools. New synthetic methodologies are developed to access these abridged scaffolds in a few steps, which allows us to rapidly optimize their biological, pharmacokinetic and pharmacodynamic properties (Chem. Soc. Rev. 2007, 36, 1432-1440).
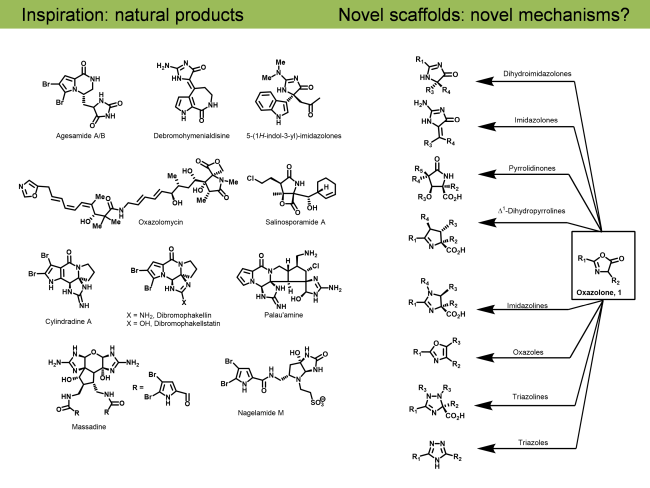
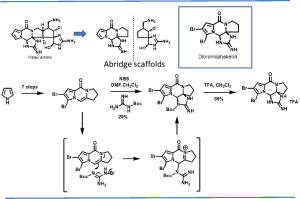 Palau’amine, phakellin & phakellstatins: The oroidin family of alkaloids is a highly diverse and complex class of biologically active secondary marine sponge metabolites containing characteristic pyrrole-2-carboxamide and 2-aminoimidazoline moieties. Other members of this family of pyrrole-imidazole marine alkaloids, including dibromophakellin and dibromophakellstatin share a similar tetracyclic framework with palau’amine. Our Chemical Biology studies identified the immunoproteasome as a cellular target of palau’amine and the phakellstatins (J. Nat. Prod. 2012, 75, 980-985). As part of these studies we reported the total synthesis of the related natural product, dibromophakellin, via a novel SN2’ type addition to olefin (Org. Lett. 2011, 13, 4550-4553) and reported its novel mechanism (Angew. Chemie Int. Ed. 2015, 54, 2830-2833).
Palau’amine, phakellin & phakellstatins: The oroidin family of alkaloids is a highly diverse and complex class of biologically active secondary marine sponge metabolites containing characteristic pyrrole-2-carboxamide and 2-aminoimidazoline moieties. Other members of this family of pyrrole-imidazole marine alkaloids, including dibromophakellin and dibromophakellstatin share a similar tetracyclic framework with palau’amine. Our Chemical Biology studies identified the immunoproteasome as a cellular target of palau’amine and the phakellstatins (J. Nat. Prod. 2012, 75, 980-985). As part of these studies we reported the total synthesis of the related natural product, dibromophakellin, via a novel SN2’ type addition to olefin (Org. Lett. 2011, 13, 4550-4553) and reported its novel mechanism (Angew. Chemie Int. Ed. 2015, 54, 2830-2833).
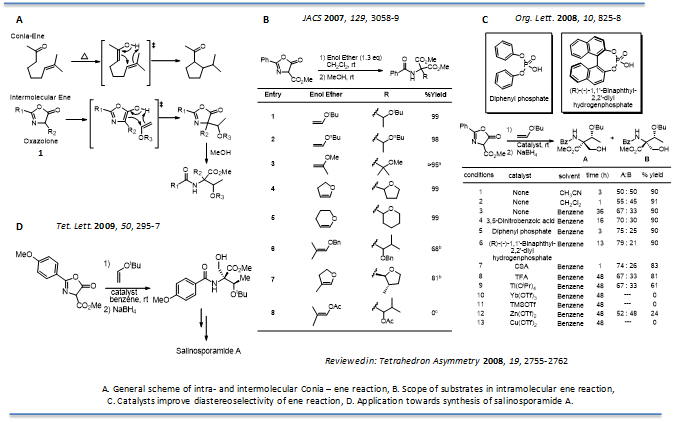
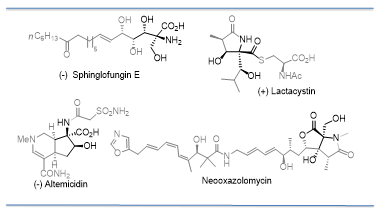 Conia-ene reactions with oxazolones: Highly substituted amino hydroxy carboxylic acids are structural features present in numerous natural products including the sphingofungins, lactacystins, altemicidines, oxazolomycins and many others (Figure 2). In order to explore their biological properties, we developed a novel Conia ene-type reactions using the oxazolone template 1. An inter-molecular version of this type of ene reaction had not been discovered yet but would greatly enhance the utility of ene reactions as an enolate alkylation alternative. We developed and reported the first intermolecular ene reaction using oxazolones (also referred to as azlactones) under very mild conditions without the use of any catalyst (J. Am. Chem. Soc. 2007, 129, 3058-3059). Oxazolones are an ideal substrates for an intermolecular ene reaction of this nature based due to the ease of formation of the aromatic enol tautomer (Tetrahedron Asymm. 2008, 19, 295-297). We subsequently optimized this reaction and found that the use of a phosphate catalyst improved the reaction rate and increased the diastereoselectivity (Org. Lett. 2008, 10, 825-828), making this reaction more useful for our total synthesis of the natural product and proteasome inhibitor, Salinosporamide A (Tet. Lett. 2009, 50, 295-297). As a clear illustration of the use of this reaction Terada and co-workers followed up shortly with the asymmetric version of this reaction, by replacing the phosphate catalyst with a chiral phosphate catalysts (Terada: J. Am. Chem. Soc. 2009, 131, 3430-3431).
Conia-ene reactions with oxazolones: Highly substituted amino hydroxy carboxylic acids are structural features present in numerous natural products including the sphingofungins, lactacystins, altemicidines, oxazolomycins and many others (Figure 2). In order to explore their biological properties, we developed a novel Conia ene-type reactions using the oxazolone template 1. An inter-molecular version of this type of ene reaction had not been discovered yet but would greatly enhance the utility of ene reactions as an enolate alkylation alternative. We developed and reported the first intermolecular ene reaction using oxazolones (also referred to as azlactones) under very mild conditions without the use of any catalyst (J. Am. Chem. Soc. 2007, 129, 3058-3059). Oxazolones are an ideal substrates for an intermolecular ene reaction of this nature based due to the ease of formation of the aromatic enol tautomer (Tetrahedron Asymm. 2008, 19, 295-297). We subsequently optimized this reaction and found that the use of a phosphate catalyst improved the reaction rate and increased the diastereoselectivity (Org. Lett. 2008, 10, 825-828), making this reaction more useful for our total synthesis of the natural product and proteasome inhibitor, Salinosporamide A (Tet. Lett. 2009, 50, 295-297). As a clear illustration of the use of this reaction Terada and co-workers followed up shortly with the asymmetric version of this reaction, by replacing the phosphate catalyst with a chiral phosphate catalysts (Terada: J. Am. Chem. Soc. 2009, 131, 3430-3431).
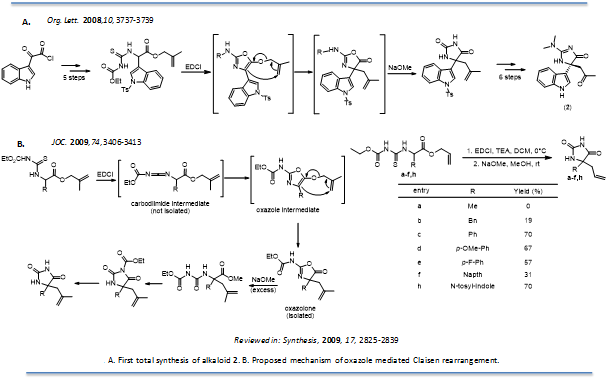
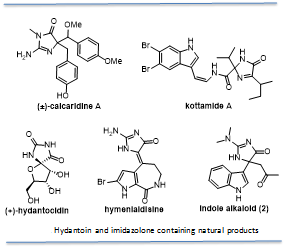 Claisen-rearrangements with oxazolones: Quaternary hydantoins and imidazol-4-ones are synthetically intriguing and are contained in a myriad of compounds that host interesting biological properties, such as (+) hydantocidin, calcaridine A, kottamides A-D and hymenialdisine, which exhibit anti-inflammatory activity. Our work in this area was focused on the unique indole marine alkaloid (2) from the tunicate Dendrodoa grossularia, whose biological activity has not yet been reported. We completed the first total synthesis of the indole alkaloid (2) (Org. Lett. 2008, 10, 3737-3739) and investigated its biological properties. As part of this total synthesis we developed a novel Claisen-type rearrangement with oxazolones (J. Org. Chem. 2009, 74, 3406-3413). In order to prepare this marine sponge metabolite, a new synthetic route needed to be constructed to access the unique 5, 5-(disubstituted)-imidazol-4-one scaffold. We hypothesized that the 5, 5-(aryl, allyl) substituted hydantoin derived from an oxazole rearrangement would be a novel and ideal approach to access the indole alkaloid 2. The hydantoin intermediate could serve as a building block to the imidazolone scaffold and act as a synthetic handle with which we could replace the carbonyl in the 2-position of the hydantoin with potentially any amine, giving access to a number of analogs if desired. In addition, the allyl group would provide easy access to the keto functionality found in the indole alkaloid (2). Overall, a novel rearrangement that gives access to a unique marine alkaloid scaffold was developed by our lab (J. Org. Chem. 2009, 74, 3406-3413). The one-pot reaction converts a thiourea into a 5, 5-(aryl, allyl) oxazolone through a new twist on the oxazole rearrangement, which upon further treatment with sodium methoxide yields a 5, 5-(aryl, allyl) hydantoin. This hydantoin was used to offer a concise synthetic route to natural product 2.
Claisen-rearrangements with oxazolones: Quaternary hydantoins and imidazol-4-ones are synthetically intriguing and are contained in a myriad of compounds that host interesting biological properties, such as (+) hydantocidin, calcaridine A, kottamides A-D and hymenialdisine, which exhibit anti-inflammatory activity. Our work in this area was focused on the unique indole marine alkaloid (2) from the tunicate Dendrodoa grossularia, whose biological activity has not yet been reported. We completed the first total synthesis of the indole alkaloid (2) (Org. Lett. 2008, 10, 3737-3739) and investigated its biological properties. As part of this total synthesis we developed a novel Claisen-type rearrangement with oxazolones (J. Org. Chem. 2009, 74, 3406-3413). In order to prepare this marine sponge metabolite, a new synthetic route needed to be constructed to access the unique 5, 5-(disubstituted)-imidazol-4-one scaffold. We hypothesized that the 5, 5-(aryl, allyl) substituted hydantoin derived from an oxazole rearrangement would be a novel and ideal approach to access the indole alkaloid 2. The hydantoin intermediate could serve as a building block to the imidazolone scaffold and act as a synthetic handle with which we could replace the carbonyl in the 2-position of the hydantoin with potentially any amine, giving access to a number of analogs if desired. In addition, the allyl group would provide easy access to the keto functionality found in the indole alkaloid (2). Overall, a novel rearrangement that gives access to a unique marine alkaloid scaffold was developed by our lab (J. Org. Chem. 2009, 74, 3406-3413). The one-pot reaction converts a thiourea into a 5, 5-(aryl, allyl) oxazolone through a new twist on the oxazole rearrangement, which upon further treatment with sodium methoxide yields a 5, 5-(aryl, allyl) hydantoin. This hydantoin was used to offer a concise synthetic route to natural product 2.