This is the end of the designated help screen>
This is the end of the designated help screen>
This is the end of the designated help screen>
You must consider the symmetry of a molecule when predicting the number of nmr signals expected. Each unique set of equivalent carbon atoms should give a characteristic signal, distinct from all other signals. The dashed red lines through the following structures indicate planes of symmetry.
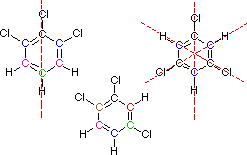
This is the end of the designated help screen>
You must consider the symmetry of a molecule when predicting the number of nmr signals expected. Each unique set of equivalent carbon atoms should give a characteristic signal, distinct from all other signals. The dashed red lines through the following structures indicate planes of symmetry.
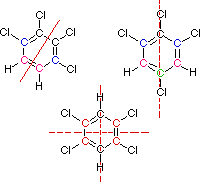
This is the end of the designated help screen>
Since these compounds all have a three carbon chain, it should be clear that the number of structurally distinct carbon atoms must be either two or three. After all, there are two end carbon atoms and one in the middle. If the end carbons are different there will be a total of three different carbons; but if the end carbons are the same there will only be two different carbons. The same analysis applies to the number of structurally distinct groups of hydrogen atoms. Additional information about the hydrogen groups may be obtained from the splitting patterns (n+1 rule).
This is the end of the designated help screen>