| A or B | } | excess H2 Pt catalyst |
C = | 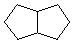 |
| Two isomeric C8H10 hydrocarbons (A & B) both give the same saturated hydrocarbon product, C, on exhaustive catalytic hydrogenation. Both A & B, but not C, show strong absorption of UV light, λmax = 245 nm. Addition of one equivalent of HBr to either A or B produces the same mixture of C8H11Br isomers (D & E). |
|