One of the most challenging aspects of learning organic chemistry is devising sequences of reactions that will lead from a designated starting compound to a desired product compound. This problem is an introduction to the planning of multistep syntheses.
For use, you have six reactant compounds (A through F); and eight reagents (1 through 8), shown below.
Following these lists, five multistep syntheses are outlined. For each of these, certain reactants or reagents must be identified by entering an appropriate letter or number in designated answer boxes.
Enter a single letter or number, indicating your choice of the best reactant or reagent, in each answer box.
Reactant Compounds: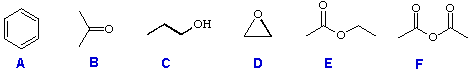
Reagents
| 1 Jones' reagent Na2Cr2O7 in H3O(+) |
2 PCC CrO3 in pyridine + HCl |
3 Sodium Hydride NaH |
4 Sodium Borohydride NaBH4 |
| 5 Thionyl Chloride SOCl2 |
6 Phosphorus Tribromide PBr3 |
7 Aluminum Trichloride AlCl3 |
8 Magnesium Mg turnings in ether |