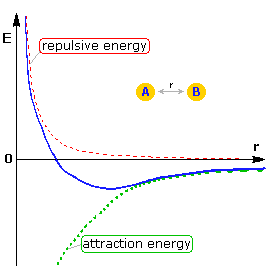
When two neutral atoms (A & B) are close enough to detect each other, they experience an attraction due to dispersion forces. The green dashed curve in the illustration on the right represents this attraction, which increases with the inverse sixth power of the distance between the atoms (r). As the atoms near each other, their outer (valence) electrons interact to repel each other, and this repulsion energy increases very rapidly as the distance r decreases. This is demonstrated by the red dashed curve in the diagram. Consequently, as the two atoms come together, an initial attraction becomes a strong repulsion, as shown by the dark blue curve. This increase in energy as atoms are crowded together is called steric repulsion or steric hindrance.
If a covalent bond forms between the atoms, the energy versus distance curve displays a distinct minimum, representing a bond energy of Eb, at a distance (req) equal to the average bond length. By clicking on the diagram, this bonding relationship will be shown as a light blue curve.