|
Name of Base (B) |
Formula of B |
Ka (BH+) |
pKa |
|---|---|---|---|
|
aliphatic nitro cpd. |
R-NO2 |
ca. 1012 |
ca. -12 |
aryl nitro cpd. |
C6H5NO2 |
ca. 1011 |
ca. -11 |
|
nitriles |
R-C≡N |
ca. 1010 |
ca. -10 |
|
aliphatic aldehydes |
R-CH=O |
ca. 108 |
ca. -8 |
|
aryl aldehydes |
C6H5CH=O |
ca. 107 |
ca. -7 |
|
aliphatic ketones |
R2C=O |
ca. 107 |
ca. -7 |
|
esters |
R-CO2R' |
ca. 3 * 106 |
ca. -6.5 |
|
aryl alkyl ethers |
C6H5OCH3 |
ca. 3 * 106 |
ca. -6.5 |
|
phenols |
C6H5OH |
ca. 3 * 106 |
ca. -6.5 |
|
aryl ketones |
C6H5COCH3 |
ca. 106 |
ca. -6 |
|
carboxylic acids |
R-CO2H |
ca. 106 |
ca. -6 |
|
sulfides |
RSR |
ca. 105 |
ca. -5 |
|
diethyl ether |
C2H5OC2H5 |
ca. 3 * 103 |
ca. -3.5 |
|
dioxane |
O(CH2CH2)2O |
ca. 103 |
ca. -3 |
|
pyran |
(CH2)5O |
ca. 3 * 102 |
ca. -2.5 |
|
tetrahydrofuran |
(CH2)4O |
ca. 102 |
ca. -2 |
|
alcohols |
R-CH2-OH |
ca. 102 |
ca. -2 |
|
aryl amides |
C6H5C(NH2)=O |
ca. 102 |
ca. -2 |
|
indole |
|
ca. 102 |
ca. -2 |
|
water |
H2O |
55 |
-1.74 |
|
aliphatic amides |
RC(NH2)=O |
3.2 |
-0.5 |
|
dimethyl sulfoxide |
(CH3)2SO |
1 |
0 |
|
phosphine oxides |
R3PO |
1 |
0 |
|
pyrrole |
C4H4NH |
1 |
0 |
|
urea |
(NH2)2C=O |
0.8 |
0.1 |
|
diphenylamine |
(C6H5)2NH |
0.15 |
0.8 |
|
p-nitroaniline |
4-O2NC6H4NH2 |
0.1 |
1.0 |
|
aniline |
C6H5NH2 |
2.5 * 10-5 |
4.6 |
|
trimethylamine N-oxide |
(CH3)3NO |
2.5 * 10-5 |
4.6 |
|
N,N-dimethylaniline |
C6H5N(CH3)2 |
10-5 |
5.1 |
|
pyridine |
C5H5N |
6.3 * 10-6 |
5.2 |
|
hydroxyl amine |
HONH2 |
1.3 * 10-6 |
5.9 |
|
2,6-dimethylpyridine |
|
2.0 * 10-7 |
6.7 |
|
imidazole |
|
10-7 |
7.0 |
|
hydrazine |
H2NNH2 |
10-8 |
8.0 |
|
alkyl phosphines |
R3P |
10-8 |
8.0 |
|
aziridine |
|
10-8 |
8.0 |
|
2,2,2-trifluoroethylamine |
CF3CH2NH2 |
5.0*10-9 |
8.3 |
|
morpholine |
O(CH2CH2)2NH |
5.0*10-9 |
8.3 |
|
DABCO |
|
K1 = 2.0 * 10-9
|
8.7
|
|
ammonia |
NH3 |
5.62 * 10-10 |
9.25 |
|
4-dimethylaminopyridine |
4-(CH3)2N-C5H4N |
2.0 * 10-10 |
9.7 |
|
ethyl amine |
C2H5NH2 |
2.0 * 10-11 |
10.7 |
|
triethyl amine |
(C2H5)3N |
1.8 * 10-11 |
10.8 |
|
diethyl amine |
(C2H5)2NH |
10-11 |
11.0 |
|
piperidine |
(CH2)5NH |
10-11 |
11.0 |
|
pyrrolidine |
(CH2)4NH |
6.3 * 10-12 |
11.2 |
|
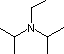
Hünig's base |
|
3.4 * 10-12 |
11.4 |
|
DBU |
|
10-12 |
12 |
|
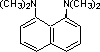
proton sponge |
|
4.4 * 10-13 |
12.3 |
|
guanidine |
(NH2)2C=NH |
2.0 * 10-14 |
13.6 |
|
pentamethylguanidine |
[(CH3)2N]2C=NCH3 |
1.8 * 10-14 |
13.8 |