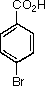
You have now made 4-bromo-3-nitrobenzoic acid from toluene in three steps.

1. Bromination and isolation of p-bromotoluene.
2. Oxidation of the methyl group to a carboxylic acid.
3. Nitration of p-bromo benzoic acid at the 3-position.
Note that the last reaction has good regiospecificity, since the ortho/para-directing bromine substituent is located in a complementary location (para) to the meta-directing and deactivating carboxyl group.