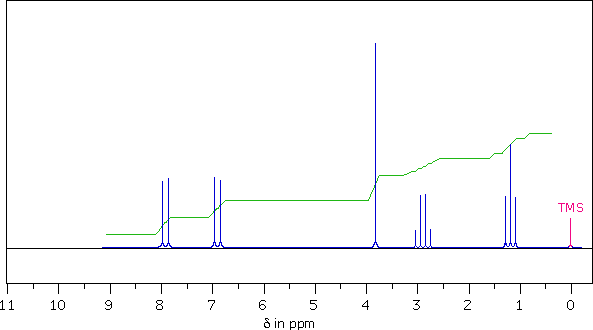
The following 1Hnmr spectrum of a C10H12O2 compound was obtained on a 90 MHz spectrometer.
A series of questions are presented below the spectrum. To answer each question enter a number or letter in the designated answer box.
Do not enter punctuation (eg. commas or semicolons) or spaces.

| 7. You should now be able to assign a structure to this C10H12O2 compound. To evaluate your assignment Click Here. |