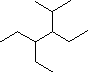
 |
In the IUPAC system what is the root or base name of this compound? | ||
| How many alkyl substituents are attached to the longest chain? | |||
| Give the IUPAC name for this compound. |
Part C: The line formula for another branched alkane is shown below.
 |
In the IUPAC system what is the root or base name of this compound? | ||
| How many alkyl substituents are attached to the longest chain? | |||
| Give the IUPAC name for this compound. |
Part D: The formula for a substituted cycloalkane is shown below.
 |
In the IUPAC system what is the root or base name of this compound? | ||
| How many alkyl substituents are attached to the ring? | |||
| In numbering the ring, which carbon (a-f) is #1? | |||
| In numbering the ring, which carbon (a-f) is #2? | Give the IUPAC name for this compound. |
Part E: The formula for another cycloalkane derivative is shown below.
 |
Give the IUPAC name for this compound. |
Part A: The line formula for a branched alkene is shown below.
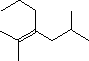 |
What is the molecular formula of this compound? | C H | |
| How many carbon atoms are in the longest chain, ignoring the double bond? | |||
| What is the longest chain incorporating both carbons of the double bond? | |||
| How many substituents are on this chain? | Give the IUPAC name for this compound. |
Part B: The line formula for a cyclic alkene is shown below.
 |
In numbering the ring, which carbon (a-g) is #1? | ||
| Give the IUPAC name for this compound. |
Part C: Name the following compounds according to the IUPAC system:
| (1) |  |
Enter the IUPAC name | ||
| (2) | 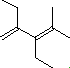 |
Enter the IUPAC name | ||
| (3) |  |
Enter the IUPAC name | ||
| (4) |  |
Enter the IUPAC name | ||
| (5) | 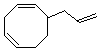 |
Enter the IUPAC name | ||
| (6) | 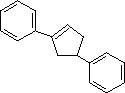 |
Enter the IUPAC name |
Part A: (CH3)2CHCH(C2H5)CH2CH2CH3
| Give the IUPAC name for this compound. |
Part B: CH2=C(C2H5)CH2CH2CH3
| Give the IUPAC name for this compound. |
Part C: (CH3)2C=CHC(C2H5)=CH2
| Give the IUPAC name for this compound. |
Part D: CH3C≡CCH(C2H5)2
| Give the IUPAC name for this compound. |
Part E: (CH3)3CC≡CCH2CH=C(CH3)2
| Give the IUPAC name for this compound. |
Name the following compounds according to the IUPAC system:
| (1) | 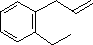 |
Enter the IUPAC name | ||
| (2) | 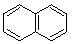 |
Enter the IUPAC name | ||
| (3) | 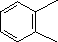 |
Enter the IUPAC name | ||
| (4) | 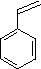 |
Enter the IUPAC name | ||
| (5) | 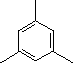 |
Enter the IUPAC name |
Name the following compounds according to the IUPAC system:
| (1) | 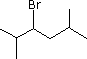 |
Enter the IUPAC name | ||
| (2) | 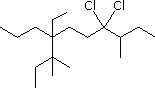 |
Enter the IUPAC name | ||
| (3) | 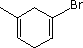 |
Enter the IUPAC name | ||
| (4) | 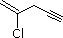 |
Enter the IUPAC name | ||
| (5) | 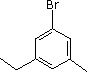 |
Enter the IUPAC name |