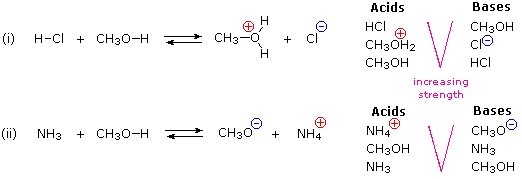
The importance of acid-base interactions in organic chemical reactions can scarcely be overemphasized. One fact that is not immediately obvious is that both acids and bases are always present together in equilibrium with each other. The following diagram illustrates this point. Methyl alcohol (CH3OH) is an amphoteric solvent, being both a weak acid and a weak base. In equation (i) the strong acid HCl is added to this solvent. In equation (ii) the base ammonia has been added to the same solvent. To the right of each equation is a list of acidic and basic components that are present in the solution. The strongest acid and the strongest base are at the top of each list.

In acid-base equilibria the weakest acid and weakest base will always be on the same side, and that will be favored. The reactants in each of these examples are amphoteric, but the weakest combination (shown at the bottom of the lists) never actually function as such, and are not shown. Equilibrium (i) lies to the right, while equilibrium (ii) is favored to the left.