|
Carbene
Complexes
Introduction
I. Benzannulation
Reaction
II.
Cyclohexadienone
Annulation
III. Tautomer
Arrested Annulation
IV. Aldol Reaction
V. Diels-Alder
Reaction
VI. Cyclobutanone
Formation
VII. Biaryl
Synthesis
VIII.
Macrocycles
Asymmetric
Catalysis
I. Ligand Design
and
Synthesis
II. Asymmetric
Diels-Alder
Reaction
III. Imino Aldol
Reaction
IV. Asymmetric
Aziridination
Synthesis of Natural Products
and Pharmaceuticals
|
|
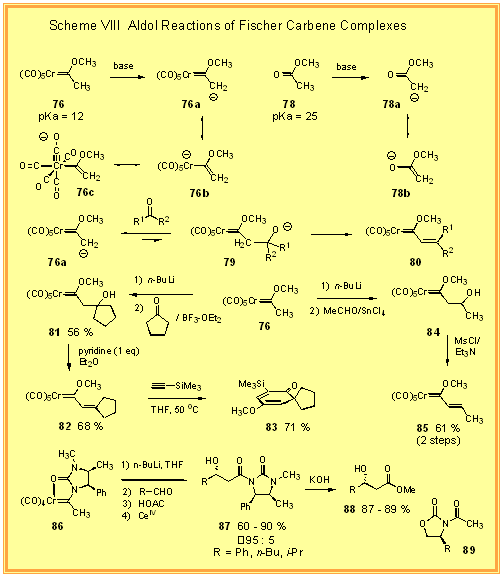
Many
reactions of Fischer carbene complexes occur with the retention
of the chromium-carbon double-bond in the product.
Most reactions in this category involve a reaction of the
carbon substituent of the carbene carbon.
One such process is the aldol reaction of alkyl carbene
complexes as illustrated in Scheme VIII.
Most of the reactions in this category have counterparts
in the |
|
chemistry
of esters. The aldol
reaction of esters is made possible by the acidity of protons
alpha to the ester carbonyl which in the case of methyl acetate
is approximately pKa = 25. The
acidity of the methyl carbene complex 76
is actually many orders of magnitude greater than an ester.
The acidity of 76 was found by Bernasconi to be pKa = 12 and is similar to a
methylene hydrogen stabilized by two carbonyl groups, i.e., a
malonate [1].[One
of the reasons for the high acidity of this complex is
undoubtedly due to the resonance delocalization of the anion
over many resonance structures including localization on the
chromium 76b and over
the five CO ligands (only one shown, 76c).
The aldol reaction of Fischer carbene complexes was first
examined by Casey and he found that, as expected for such a
stable anion, the addition of 76a to a carbonyl compound was unfavorable (76a to 79) [2].iThe
only examples where addition products could be observed were
when the elimination occurred to give unsaturated complexes
of the type 80.
This could drive the unfavorable equilibrium between 76a
and 79 such that reasonable yields of 80 could be realized. The
formation of unsaturated complexes by this method was limited to
aryl aldehydes and non-enolizable aldehydes.
We were able to isolate aldol addition products
from these reactions for the first time if |
 |
|
Lewis
acids were used to activate the carbonyl compound towards
addition of the enolate of the carbene complex.
The best Lewis acid for the addition to ketones is boron
trifluoride – etherate [3][iand
the best Lewis acid for the addition to aldehydes is tin
tetrachloride [4].iOne
of the important applications of this aldol reaction is in the
preparation of unsaturated carbene complexes which are
starting materials for the benzannulation and cyclohexadienone
annulation reactions (vide
supra) and for the Diels-Alder reaction of carbene complexes
(vide infra). As an example, the reaction of the complex 82, derived from cyclopentenone, with trimethylsilylacetylene gives
the spirocyclohexadienone 83
in 71 % yield [3].
Asymmetric aldol reactions are also possible with chiral
imidazolidinone complexes of the type 86 [5].[The anion derived from 86 is much more reactive and its addition to carbonyls occurs
without the need for Lewis acid activation.
The aldol addition of 86
occurs to a variety of aldehydes with greater than 95 : 5 facial
selectivity on the aldehyde. Workup
of the reaction includes an oxidative removal of the metal to
give the aldol adduct 87
from which can be liberated optically pure b-hydroxy acids of
the type 88. It should be pointed out that the Evans oxazolidinone 89
does not give high selectivity in aldol reactions.
The Evans aldol reaction is limited alpha-substituted enolate
equivalents. |
[1]
Bernasconi, C. F.; Leyes, A. E.; Ragains, M. L.; Shi, Y.; Wang, H.;
Wulff, W. D., J. Am. Chem. Soc.,
1998, 120, 8632.
[2]
Casey, C. P.; Boggs, R. A.; Anderson, R.
L., J. Am. Chem. Soc., 1972,
94, 8947.
[3]
Wulff, W. D.; Gilbertson, S. R.; J.
Am. Chem. Soc., 1985,
107, 503.
[4]
Wang, H.; Hsung, R. P.; Wulff, W. D., Tetrahedron
Lett., 1998, 39,
1849.
[5]
Powers, T. S.; Shi, Y.; Wilson, K. J.; Wulff, W. D., J.
Org. Chem., 1994, 59,
6882.
|
