Chemical Biology
The structural diversity of marine sponge metabolites makes them ideal as tools to discover novel targets and mechanisms. In our chemical biology lab, students will unravel the biological properties of marine sponge metabolites and determine their use as therapeutic leads or biochemical tools.
THE PROTEASOME: Proteins undergo constant proteolytic degradation to regulate intracellular processes and maintain biological homeostasis. One of the main proteolytic pathways involves the proteasome, which is responsible for the degradation of misfolded, oxidatively damaged and redundant proteins. Modulation of proteasome (i.e. inhibition or activation) represents a therapeutic approach to treat various diseases.
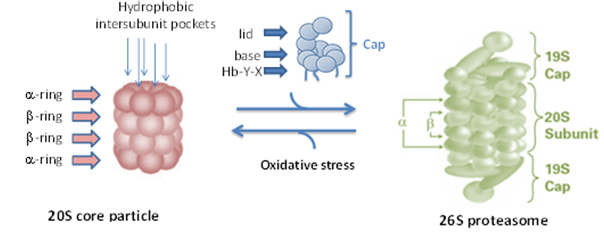
NEW ! ACTIVATION of the PROTEASOME: As we age, proteasome activity declines and oxidative protein damage increases, resulting in the accumulation of misfolded, oxidatively damaged proteins. When protein accumulation exceeds cellular clearance in neurons, aggregation occurs, which is the hallmark of several neurological disorders, including Parkinson’s disease (PD), Alzheimer’s disease (AD), Huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS). Although treatments are available that temporarily address the symptoms of neurological disorders, there are no treatment options that prevent, slow or reverse the pathological aggregation of proteins that induce neurodegenerative disorders. Our group has discovered several novel classes of molecules that directly enhance only 20S proteasome activity and induce the degradation of a-synuclein (a major pathological protein involved in Parkinson’s disease) and tau (a major pathological protein involved in Alzheimer’s disease) and clear oxidatively damaged proteins from cells. These compounds represent one of the first small molecules capable of directly enhancing 20S proteolytic activity by inducing an open-gate conformation (i.e. active form) of the 20S proteasome. Work in our group is focused on characterizing the details and therapeutic implications of this new type of proteasome regulation.
INHIBITION of the PROTEASOME: Proteasome inhibition is the standard of care for the treatment of multiple myeloma (MM, blood-type cancer). The proteasome inhibitor bortezomib is approved for the treatment of MM and relapsing mantle cell lymphoma (MCL). Second-generation MM drugs include the proteasome inhibitors carfilzomib (recently FDA approved for MM), NPI-0052 (salinosporamide A), ixazomib, CEP-18770, and ONX0912, which are at different stages of clinical development. All these proteasome inhibitors operate via the same mechanism of action (competitive binding in the catalytic binding sites). Unfortunately, nearly all patients become intolerant or resistant, after which the average survival time is <1 year. Students in our group are focused on discovering proteasome inhibitors that operate via novel mechanisms of actions to overcome this resistance to current leading drugs.
Several new classes of proteasome inhibitors have been discovered by our group:
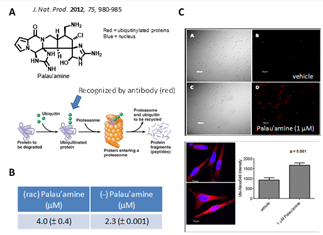
- 1. The oroidin family of alkaloids is a highly diverse and complex class of biologically active secondary marine sponge including dibromophakellin and dibromophakellstatin share a similar tetracyclic framework with palau’amine. We discovered that these compounds inhibit the human proteasome resulting in the accumulation of ubiquitinylated (red) protein substrates (J. Nat. Prod. 2012, 75, 980-985). An X-ray co-crystal structure of the natural product mimic indolo-phakellins bound to the human proteasome indicated that these agents are mechanistically unique, in that they are b5-specific noncovalent proteasome inhibitors (Angewandte Chemie Int. Ed. 2015, 54, 2830-2833).
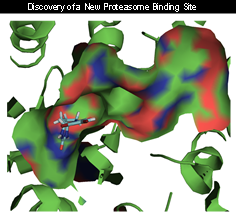
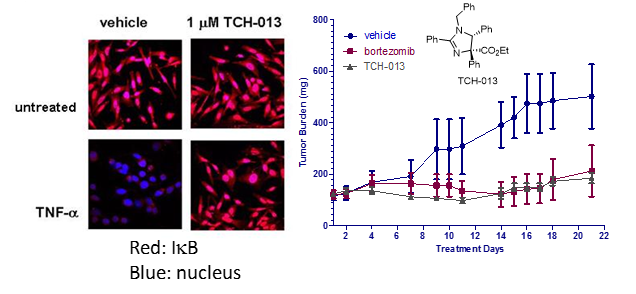
- 2. Allosteric regulation of the proteasome was discover using the small imidazoline scaffold, TCH-013. Consistent with a mechanism distinct from competitive inhibitors, TCH-013 acts additively with and overcomes resistance to bortezomib. Importantly, TCH-013 induces apoptosis in a panel of myeloma and leukemia cell lines, but in contrast, normal lymphocytes, primary bone marrow stromal cells and macrophages are resistant to its cytotoxic effects. TCH-013 was equally effective in blocking MM cell growth in co-cultures of MM cells with hBMSC isolated from CD138 negative BM samples of MM patients. The cellular activity translated well in vivo where TCH-013 delayed tumor growth in a MM xenograft mouse model to a similar extent as bortezomib.
- 3. In collaboration with the Odom laboratory we found that substituted quinolines interact as noncovalent inhibitors of the 20S proteasome via novel mechanism of action. (Bioorganic and Medicinal Chemistry, 2016, 24, 2441-2450).
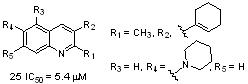
- 4.4. In collaboration with Gaczynska laboratory (Univ. Texas
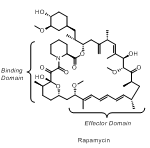
Health Science Center San Antonio) we are preparing allosteric modulators based on the natural product rapamycin.