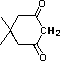|
Common Name |
Formula |
Acidity Constant |
pKa |
trifluoromethanesulfonic acid |
CF3SO3H |
ca. 1013 |
ca. -13 |
benzenesulfonic acid |
C6H5SO3H |
ca. 103 |
ca. -2.5 |
methanesulfonic acid |
CH3SO3H |
ca. 3 * 102 |
ca. -2.0 |
trifluoroacetic acid |
CF3CO2H |
1.0 |
0.0 |
picric acid |
(O2N)3C6H2OH |
0.5 |
0.3 |
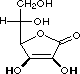
squaric acid |
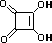 |
K1 = 0.33 |
1.5 |
|
trichloroacetic acid |
CCl3CO2H |
0.23 |
0.77 |
oxalic acid |
(CO2H)2 |
K1 = 6.5 * 10-2
|
1.2 |
|
dichloroacetic acid |
CHCl2CO2H |
5.5 * 10-2 |
1.25 |
|
fluoroacetic acid |
FCH2CO2H |
2.5 * 10-3 |
2.6 |
|
chloroacetic acid |
ClCH2CO2H |
1.36 * 10-3 |
2.87 |
|
citric acid |
C(OH)(CH2CO2H)2CO2H |
K1 = 7.4 * 10-4
|
3.13 |
|
formic acid |
HCO2H |
1.77 * 10-4 |
3.75 |
ascorbic acid |
|
K1 = 6.7 * 10-5
|
4.17 |
|
benzoic acid |
C6H5CO2H |
6.3 * 10-5 |
4.20 |
|
acetic acid |
CH3CO2H |
1.77 * 10-5 |
4.75 |
|
thiophenol |
C6H5SH |
2.5 * 10-7 |
6.6 |
|
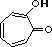
tropolone |
|
2.0 * 10-7 |
6.7 |
|
p-nitrophenol |
O2NC6H4OH |
5.7 * 10-8 |
7.2 |
|
peracetic acid |
CH3COO2H |
5.7 * 10-9 |
8.2 |
|
succinimide |
(CH2CO)2NH |
2.5 * 10-10 |
9.6 |
|
phenol |
C6H5OH |
10-10 |
10.0 |
|
chloral hydrate |
CCl3CH(OH)2 |
10-10 |
10.0 |
|
benzenesulfonamide |
C6H5SO2NH2 |
8 * 10-11 |
10.1 |
|
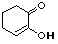
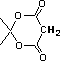
2-hydroxy-2-cyclohexenone |
|
5.0 * 10-11 |
10.3 |
|
ethanethiol |
C2H5SH |
2.5 * 10-11 |
10.6 |
|
acetoxime |
(CH3)2C=NOH |
6 * 10-13 |
12.2 |
|
2,2,2-trifluoroethanol |
CF3CH2OH |
4 * 10-13 |
12.4 |
|
imidazole |
C3H3N2H |
3.3 * 10-15 |
14.5 |
|
pyrrole |
C4H4NH |
10-15 |
15 |
|
ethanol |
C2H5OH |
10-16 |
16 |
|
1°-amides |
RCONH2 |
10-17 |
17 |
|
p-nitroaniline |
O2NC6H4NH2 |
3.3 * 10-19 |
18.5 |
t-butanol |
(CH3)3COH |
10-19 |
19 |
aniline |
C6H5NH2 |
10-27 |
27 |
1,1,1,3,3,3,-hexamethyldisilazane |
[(CH3)3Si]2NH |
10-30 |
30 |
pyrrolidine |
C4H8NH |
10-32 |
32 |
diisopropylamine |
[(CH3)2CH]2NH |
1.9 * 10-36 |
35.7 |
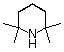
tetramethylpiperidine |
|
10-37 |
37 |
Michigan State University |