CHEMISTRY
AT THE INTERFACE
BIOMIMETIC
AND NANOPOROUS INTERFACES
Blanchard
group research is focused on three problems of interest to
both the analytical chemistry and materials science
communities:
- Design
and characterization of molecular monolayer and
multilayer assemblies
- Understanding
energy exchange processes between dissimilar molecules
- Achieving
a molecular understanding of how confinement and
immobilization affect the ability for a catalytic
species to function
We
use picosecond nonlinear laser spectroscopies in
conjunction with more traditional methods to address
these problems. Learn more about our picosecond laser
instruments here or by clicking on the
"Techniques & Instrumentation" page above.
|
CONTROLLING
INTERFACIAL FLUIDITY
Covalently
bound interfacial adlayers are not fluid, and fluid
adlayers are not physically or chemically robust. These
limiting cases have frustrated advances in fields such as
molecular-scale lubrication, chemical separations and
cellular adhesion.
We are developing a novel family of interfaces that can be
bound to a surface and at the same time retain the
properties of a fluid. Both the thermodynamic driving
force for complexation and the kinetics of surface
diffusion can be controlled through metal ion
complexation, system pH, surface complexing moieties, and
the amphiphile head group. |
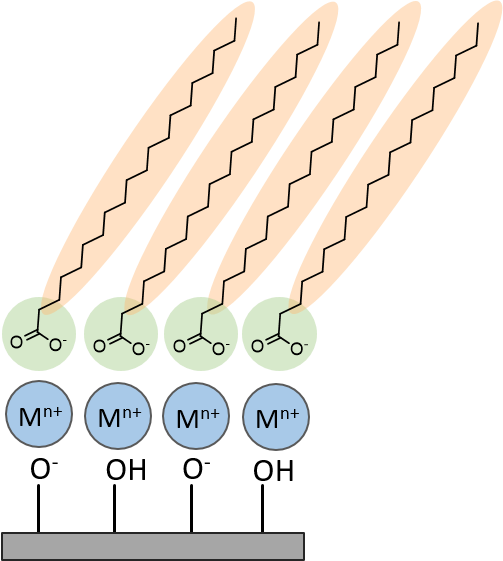
Example
amphiphilic monolayer with metal ion complexation on a
solid support.
|
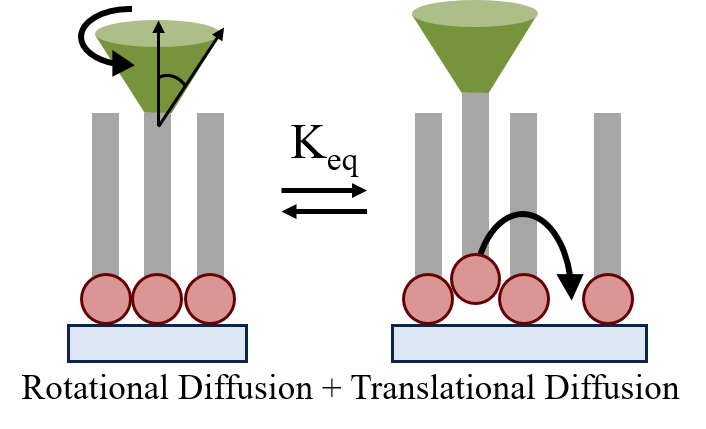
| Diffusional
Motion as a Gauge of Interfacial Adhesion and Fluidity |
|
|
Combining
two well known relationships for the translational and
rotational diffusion behavior of a molecular in a
homogeneous environment, we have derived an equation
which allows for the comparison between theoretically
derived diffusional behavior to experimentally measure
rotational and translational diffusion values. The
utilization of this relationship allows for quantitative
characterization of interfacial adhesion strengths and a
measure of heterogeneity depending upon derivation from
the expected theoretical values.
Comparing
the measured translational diffusion to the effective
translational diffusion, derivded from the combination
of the Debeye-Stokes-Einstein-Sutherland relationships
and the measured rotational diffusion, allows for the
determination of the equilibrium binding constant of the
film to the supporting surface. The equilibrium binding
constant ultimately allows for the quantitative
determination of the interfacial adhesion of the
supported film.
|
Controlling
Interface Organization Using Metal-Ion Oxidation State Toggling
Several well-established means of binding monolayers to
surfaces in a robust manner (e.g., tiol-gold
interactions, ionic complexation chemsitry) involve
surface-monolayer interactions that are fixed, and in many
of these instances the means of attachment determines the
organization of the resulting monolayer. Tthe inability to
change the binding properties of these films
post-deposition can limit their applications.
We aim to address this problem by investigating the
possibility of controlling interfaces and interfacial
properties after a monolayer or film has been deposited
onto a solid substrate, specifically, controlling in
situ and reversibly the manner in which a layer or
film binds to a surface. We plan to electrochemically
toggle the oxidation state of the metal ions in a metal
ion-complexed monlayer and explore the consequences of
this process on the organization of that layer on the
surface.
The ability to change the oxidation state and create
monolayers with controllable properties after deposition
is advantageous for applications such as stationary phases
in chemical separations, films with selective and
controllable permeability, or surfaces with controllable
optical properties, for example. |
|
Copper-complexed
monolayer with an initial oxidation state (2+, top)
toggled to a different oxidation state (1+, bottom).
|
INTERFACIAL CHARGE
GRADIENTS
|
Room
temperature ionic liquids (RTILs) are particularly
intriguing due to the wide temperature range in which they
exist in the liquid phase. Additionally, they exhibit a
wide redox window and are able to dissolve both polar and
non-polar species. We are investigating the fundamental
properties of ionic liquids to understand the dynamics,
organization, and response of these materials to external
forces. Once these properties are more fully understood,
the systems can be applied to various uses, such as
solvents for organic synthesis, electrolytes in
supercapacitors, and electro-optical materials.
|
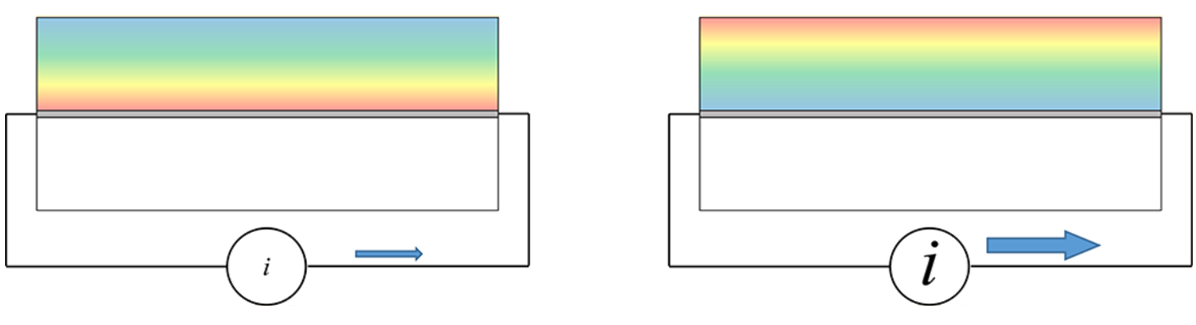
Modulating
Induced Charge Density Gradients
|
We
have observed direct evidence for charge-induced
long-range (ca. 100 μm) order in the RTIL
1-butyl-3-methylimidazolium tetrafluoroborate, supported
on a silica surface. We have measured the rotational
diffusion dynamics of anionic, cationic, and neutral
chromophores as a function of distance from a silica
surface. The results reflect the excess charge density
gradient induced in the RTIL by the (negative) charge
present on the silic asurface. Identical measurements in
ethylene glycol reveal spatially invariant reorientation
dynamics for all chomophores. Capping the silica support
with dimethyldichlorosilane (silanization) results in
spatially invariant dynamics in the RTIL.
We have further demonstrated experimental control over the
sign and magnitude of an induced charge density gradient,
ρf, in the RTIL BMIM+BF4-.
The spatial extent of ρf was characterized through
the rotational diffusiontime constant gradient of a
cationic chromophore in the RTIL. The sign and magnitude
of ρf in BMIM+BF4-
is linked directly to the surface chargedensity of the
electrode, which can be controlled. ITO and FTO are used as
solid supports and transparent conductive electrodes during
these experiments to demonstrate this control. |
BIOMIMETIC
INTERFACES
The
creation of interfacial structures that can function
biomimetically is a gateway to the design of biosensors.
We have been actively involved in the creation of such
structures where we deposit a lipid bilayer structure,
with fluorescent and electrochemical probes embedded at
specific locations within the interface. Our efforts are
aimed at probing local organization and fluidity in the
bilayer, so that we will be able to incorporate
transmembrane proteins into these interfaces for use as
sensing elements.
|
|
Idealized
structure of a supported lipid bilayer showing the
surface binding layer o substrate (pink), hydrophilic
polymer layer (yellow), lipid connecting layer (blue),
and the outer and inner lipid leaflets (green).
|
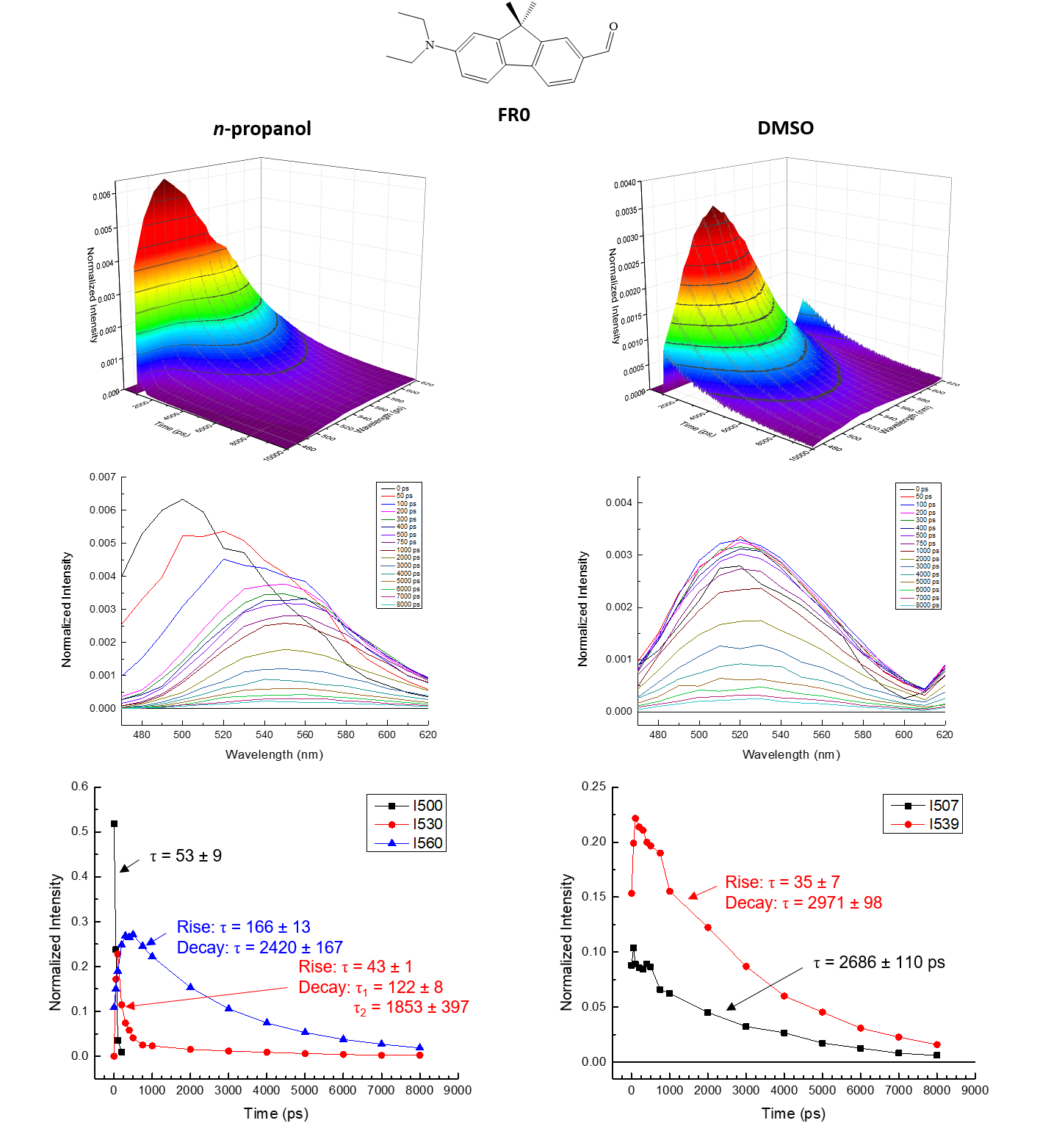
PHOTO-ACTIVATED
PRECISION CHEMISTRY
Super-photobase
molecules can be utilized in various applications such as
precision chemistry and high-speed chemical sensing. Upon
excitation with electromagnetic radiation, reactivity of
such compounds is activated (pKa > 20). Precursor
molecules of these photoactive compounds also exhibit
distinct properties, such as unique solvent-dependent
excited-state behavior. Current work focuses on
characterizing the excited-state behaviorFR0 of these
molecules using time-correlated single photon counting
(TCSPC). Results have indicated that certain percursor
molecules exhibit spectral shift dynamics and multiple
excited-state populations resulting from interactions with
surrounding solvent molecules. Specific factors
influencing this unique behavior are currently being
investigated, including proton exchange, solvent
viscosity, and solvent hydroxyl group concentration. Using
TCSPC, the spectral shift dynamics across a particular
wavelength range can be determined in protic vs aprotic
solvents, as well as the population densities of the
various excited states of such precursor molecules (see
figure).
|
|
|

